42558-54-3
- Product Name:Methyl isobutyrylacetate
- Molecular Formula:C7H12O3
- Purity:99%
- Molecular Weight:144.17
Product Details;
CasNo: 42558-54-3
Molecular Formula: C7H12O3
Appearance: colorless to yellowish liquid
Best Quality Chinese Factory Supply Methyl isobutyrylacetate 42558-54-3 Efficient Transportation
- Molecular Formula:C7H12O3
- Molecular Weight:144.17
- Appearance/Colour:colorless to yellowish liquid
- Vapor Pressure:1.161mmHg at 25°C
- Melting Point:-75 °C
- Refractive Index:1.4265
- Boiling Point:185.8 °C at 760 mmHg
- PKA:10.59±0.46(Predicted)
- Flash Point:68.9 °C
- PSA:43.37000
- Density:0.993 g/cm3
- LogP:0.77460
Methyl isobutyrylacetate(Cas 42558-54-3) Usage
|
Chemical Properties |
colorless to yellowish liquid |
|
Uses |
Methyl isobutyrylacetate is used for the synthesis if heterocycles (Furane, Pyrazolone, Chinilone) and building block for pharmaceuticals (reducer of cholesterol). Product Data Sheet |
|
Flammability and Explosibility |
Notclassified |
InChI:InChI=1/C7H12O3/c1-4-6(8)10-7(9)5(2)3/h5H,4H2,1-3H3
42558-54-3 Relevant articles
-
Wallingford,Homeyer,Jones
, p. 2254 (1941)
-
Synthetic method of methyl isobutyrylacetate
-
Paragraph 0016-0027, (2021/05/29)
The invention belongs to the technical f...
Cu-Mediated Expeditious Annulation of Alkyl 3-Aminoacrylates with Aryldiazonium Salts: Access to Alkyl N2-Aryl 1,2,3-Triazole-carboxylates for Druglike Molecular Synthesis
Liu, Hao-Nan,Cao, Hao-Qiang,Cheung, Chi Wai,Ma, Jun-An
supporting information, p. 1396 - 1401 (2020/02/22)
Alkyl N-aryl 1,2,3-triazole-carboxylates...
Chiral Vanadyl(V) Complexes Enable Efficient Asymmetric Reduction of β-Ketoamides: Application toward (S)-Duloxetine
Chen, Chien-Tien,Maity, Nabin Ch.,Agarwal, Rachit,Lai, Chien-Fu,Liao, Yiya,Yu, Wei-Ru
supporting information, p. 6408 - 6419 (2020/07/14)
High-valent chiral oxidovanadium(V) comp...
Preparation method of atorvastatin calcium
-
Paragraph 0012; 0013, (2018/07/30)
The invention belongs to the technical f...
42558-54-3 Process route
-
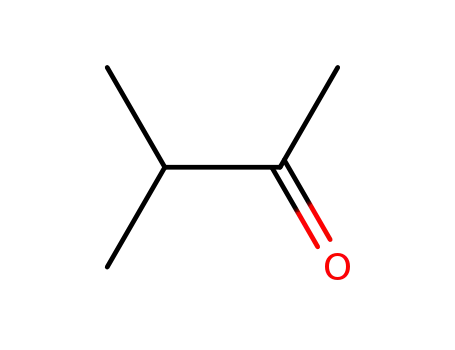
- 563-80-4
3-methyl-butan-2-one

-
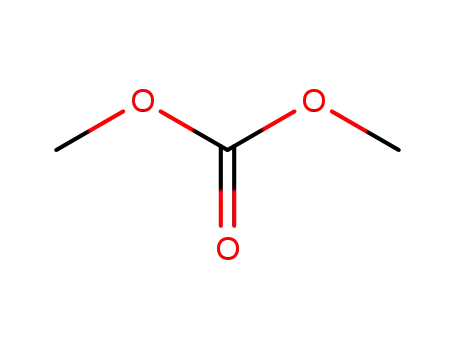
- 616-38-6
carbonic acid dimethyl ester

-
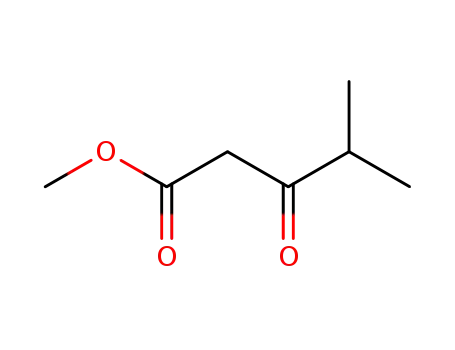
- 42558-54-3
Methyl 4-methyl-3-oxopentanoate
| Conditions | Yield |
|---|---|
|
3-methyl-butan-2-one; With sodium hydride; In tetrahydrofuran; for 0.333333h;
carbonic acid dimethyl ester; In tetrahydrofuran; at 30 ℃; Further stages.;
|
85% |
|
3-methyl-butan-2-one; carbonic acid dimethyl ester; With sodium hydride; In toluene; at 80 ℃; for 5h;
With water; acetic acid; In toluene;
|
62.72% |
|
carbonic acid dimethyl ester; With sodium hydride; In toluene; Reflux; Inert atmosphere;
3-methyl-butan-2-one; In toluene; Reflux; Inert atmosphere;
|
|
|
With sodium hydride; In toluene; Reflux;
|
|
|
With sodium hydride; In toluene; mineral oil; Reflux; Inert atmosphere;
|
|
|
With sodium hydride; In toluene; Inert atmosphere; Reflux;
|
|
|
With potassium tert-butylate; In tetrahydrofuran; at 60 ℃; for 8h; Inert atmosphere;
|
42 g |
|
With sodium hydride; In toluene; Inert atmosphere; Reflux;
|
|
|
With sodium hydride; In toluene; at 110 ℃; Inert atmosphere;
|
-

- 563-80-4
3-methyl-butan-2-one

-

- 616-38-6
carbonic acid dimethyl ester

-
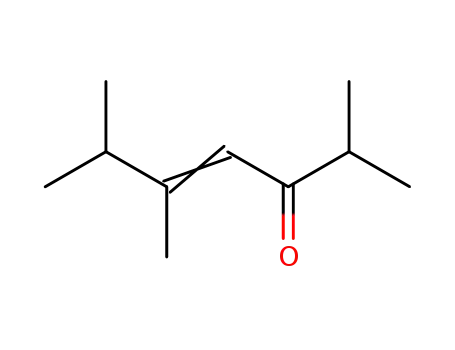
- 16466-21-0
2,5,6-trimethylhept-4-en-3-one

-
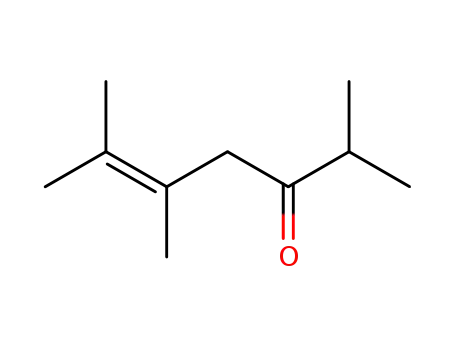
- 101459-87-4
2,5,6-trimethylhept-5-en-3-one

-

- 42558-54-3
Methyl 4-methyl-3-oxopentanoate
| Conditions | Yield |
|---|---|
|
3-methyl-butan-2-one; With sodium hydride; In 1,4-dioxane; mineral oil; at 20 ℃; for 0.166667h;
carbonic acid dimethyl ester; In 1,4-dioxane; mineral oil; at 50 ℃; for 3h; Reagent/catalyst; Solvent; Temperature; Time;
|
88% |
42558-54-3 Upstream products
-
563-80-4

3-methyl-butan-2-one
-
124-41-4
sodium methylate
-
105-58-8
Diethyl carbonate
-
186581-53-3

diazomethane
42558-54-3 Downstream products
-
59742-51-7
methyl 2,4-dimethyl-3-oxopentanoate
-
130954-89-1
methyl 4-(4-fluorophenyl)-2-(1-methylethyl)-3-quinolinecarboxylate
-
123184-09-8
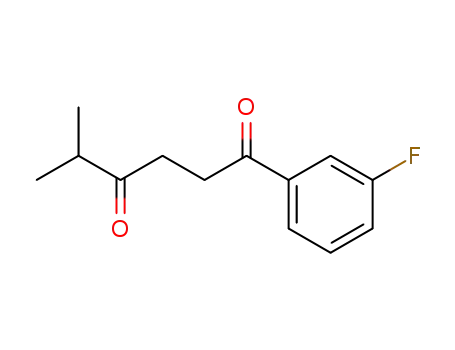
1-(3-Fluoro-phenyl)-5-methyl-hexane-1,4-dione
-
123184-13-4
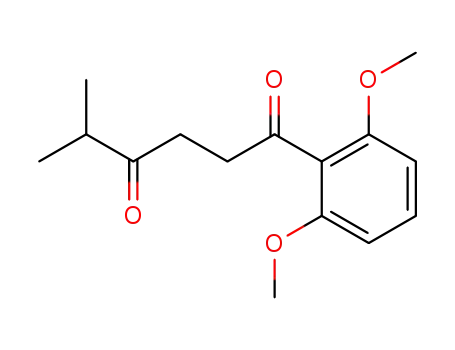
1-(2,6-Dimethoxy-phenyl)-5-methyl-hexane-1,4-dione
Relevant Products
-
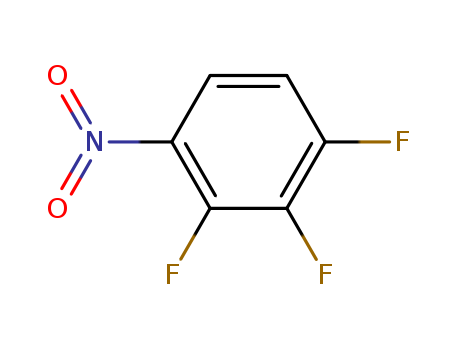
1,2,3-Trifluoro-4-nitrobenzene
CAS:771-69-7
-
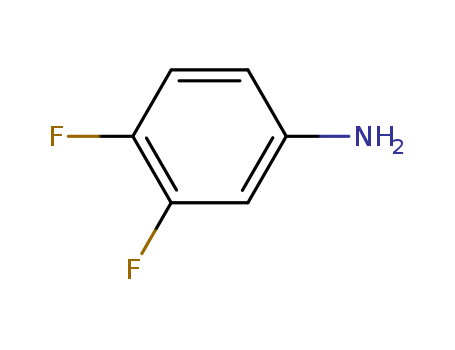
3,4-Difluoroaniline
CAS:3863-11-4
-
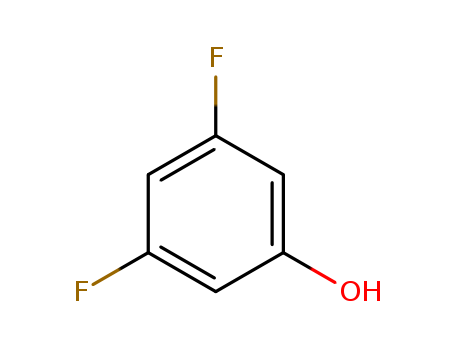
3,5-Difluorophenol
CAS:2713-34-0