211427-08-6
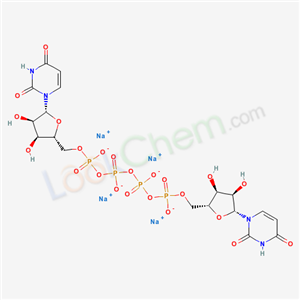
- Product Name:Diquafosol tetrasodium
- Molecular Formula:C18H22N4Na4O23P4
- Purity:99%
- Molecular Weight:878.2344
Product Details;
CasNo: 211427-08-6
Molecular Formula: C18H22N4Na4O23P4
Export Chinese Manufacturer Supply Diquafosol tetrasodium 211427-08-6 Fast Shipping
- Molecular Formula:C18H22N4Na4O23P4
- Molecular Weight:878.2344
- Boiling Point:°Cat760mmHg
- Flash Point:°C
- PSA:455.01000
- Density:g/cm3
- LogP:-2.43740
INS 365(Cas 211427-08-6) Usage
|
Description |
Diquafosol (INS-365) was approved in Japan in 2010 as a 3% ophthalmic solution for treatment of dry eye disease. Clinical diagnosis of dry eye is difficult because the condition presents a variety of symptoms. Treatment options include tear supplements (lubricants), anti-inflammatory drugs (e.g., cyclosporine eye drops or steroid eye drops), and tear retention devices. Diquafosol is a unique agent for the treatment of dry eye in that it acts as a P2Y2 purinergic receptor agonist with the ability to activate this receptor on the ocular surface and stimulate water, lipid, and mucin secretion. Diquafosol is metabolized by phosphodiesterases to UTP, UDP, UMP, and uridine. Diquafosol has been well tolerated in clinical trials, with side effects being local to the ocular surface.In addition,no serious ocular or systemic adverse drug reactions were found during the clinical trials. |
|
Originator |
Inspire Pharmaceuticals (United States) |
|
Uses |
Enhancement of mucosal hydration in the treatment of chronic dry eye (P2Y2 receptor antagonist). |
|
Brand name |
Diquas |
|
Clinical Use |
Diquafosol tetrasodium was approved in April 2010 as Diquas ? ophthalmic solution 3% for the treatment of dry eye syndrome and launched in Japan by Santen Pharmaceuticals. Diquafosol tetrasodium was originally discovered by Inspire Pharmaceuticals. In 2001, it was licensed to Santen for co-development and commercialization in Asian countries, and co-developed in collaboration with Allergan for the countries outside of Asia. In the U.S., diquafosol tetrasodium was submitted for a New Drug Application (NDA) as Prolacria ?(2% ophthalmic formulation) in June 2003. However, it is still in Phase III clinical development for dry eye syndrome. Diquafosol tetrasodium, also known as INS- 365, is a P2Y2 receptor agonist, which activates P2Y2 receptor on the ocular surface, leading to rehydration through activation of the fluid pump mechanism of the accessory lacrimal glands on the conjunctival surface. |
|
Synthesis |
The large-scale synthesis route of diquafosol tetrasodium is described in Scheme 4. Commercially available uridine 5’-diphosphate disodium salt (21) was transformed into the corresponding tributylamine salt by ion exchange chromatography on Dowex 50 using Bu3NH+ phase, and then dimerized by means of CDI in DMF at 50 oC. The crude product was purified by Sephadex DEAE column followed by ion exchange using a Dowex 50W resin in Na+ mode. The onepot process provided diquafosol tetrasodium (IV) in 25% yield. |
InChI:InChI=1/C18H26N4O23P4.4Na/c23-9-1-3-21(17(29)19-9)15-13(27)11(25)7(41-15)5-39-46(31,32)43-48(35,36)45-49(37,38)44-47(33,34)40-6-8-12(26)14(28)16(42-8)22-4-2-10(24)20-18(22)30;;;;/h1-4,7-8,11-16,25-28H,5-6H2,(H,31,32)(H,33,34)(H,35,36)(H,37,38)(H,19,23,29)(H,20,24,30);;;;/q;4*+1/p-4/t7-,8-,11-,12-,13-,14-,15-,16-;;;;/m1..../s1
Relevant Products
-
ADENOSINE 5'-DIPHOSPHORIBOSE SODIUM SALT
CAS:68414-18-6
-
Sitagliptin phosphate
CAS:654671-77-9
-
Cefradine
CAS:38821-53-3