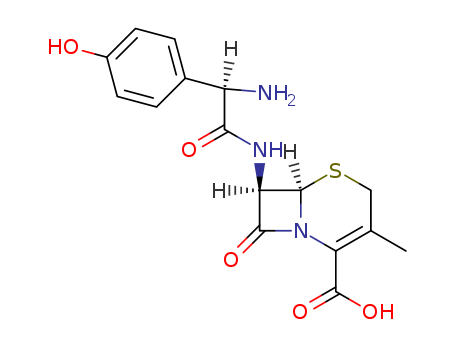
50370-12-2
- Product Name:Cefadroxil
- Molecular Formula:C16H17N3O5S
- Purity:99%
- Molecular Weight:363.394
Product Details;
CasNo: 50370-12-2
Molecular Formula: C16H17N3O5S
Top Purity Quality Manufacturer Supply Cefadroxil 50370-12-2 Best Price
- Molecular Formula:C16H17N3O5S
- Molecular Weight:363.394
- Vapor Pressure:2.71E-26mmHg at 25°C
- Melting Point:197°C (rough estimate)
- Refractive Index:1.728
- Boiling Point:789.861 °C at 760 mmHg
- PKA:3.12±0.50(Predicted)
- Flash Point:431.492 °C
- PSA:158.26000
- Density:1.592 g/cm3
- LogP:1.17960
Cefadroxil(Cas 50370-12-2) Usage
|
Description |
Cephadroxil is an orally administered antibiotic, which is very similar to cephalexin. After oral administration, its peak serum level is slightly lower than that of cephalexin, but it is excreted more slowly. Therefore, it can be used for oral administration at 8- or 12-hourly intervals (Buck and Price, 1977; Pfeffer et al., 1977). Cephadroxil is still available in a number of European countries (for example, Spain, Belgium, Luxembourg, Portugal, and Finland, among others). |
|
Chemical Properties |
Cefadroxil is a light orange colored powder. It is a cephalosporin antibiotic and is used for the treatment of bacterial infections. Cefadroxil is stable under recommended storage conditions. Cefadroxil is not considered hazardous when handled under normal medical conditions and with good housekeeping |
|
Originator |
Oracefal,Bristol,France,1977 |
|
Uses |
Antibacterial;Bacterial transpeptidase inhibitor |
|
Definition |
ChEBI: A cephalosporin bearing methyl and (2R)-2-amino-2-(4-hydroxyphenyl)acetamido groups at positions 3 and 7, respectively, of the cephem skeleton. |
|
Manufacturing Process |
1.8 g of sodium N-(1-methoxycarbonyl-1-propen-2-yl)-D(-)-α-amino-(4- hydroxyphenyl)acetate was suspended in 10 ml of acetone, and one droplet of N-methylmorpholine was added thereto, and the mixture was cooled to -15°C.There was added 0.85 g of ethyl chlorocarbonate thereto, and the mixture was reacted at -13°C to -10°C for 30 minutes, and then the reaction solution was cooled to -20°C.On the other hand, 1 g of 7-amino-3-methyl-3-cephem-4-carboxylic acid was suspended in 20 ml of methanol, and 1.4 g of triethylamine was added thereto to be dissolved, and 0.4 ml of acetic acid was further added thereto. This solution was cooled to -20°C and the mixed acid anhydride prepared previously was added thereto. After the mixture was reacted at -20°C for 1 hour, the temperature of the reaction mixture was raised to 0°C over a period of 1 hour, and the mixture was reacted for 3 hours at the same temperature.After the reaction, 1 ml of water was added to the reaction mixture, and the mixture was adjusted to a pH of 1.0 with concentrated hydrochloric acid while being cooled, and then stirred for 30 minutes, The insoluble matters were filtered off, and the filtrate was adjusted to a pH of 5.5 with triethylamine. This solution was concentrated under reduced pressure, and the residue was diluted with 20 ml of acetone to precipitate white crystals. The crystals were collected by filtration and washed with ethanol to obtain 1.46 g of white crystals of 7-[D(-)-α-amino-(4-hydroxyphenyl)acetamido]-3-methyl-3- cephem-4-carboxylic acid having a decomposition point of 197°C. |
|
Therapeutic Function |
Antibacterial |
|
Health Hazard |
Exposures to cefadroxil cause certain common side effects. These include nausea, vomiting, stomach disorders, rashes, itching, unusual tiredness or weakness, yellowing of the skin or eyes, red, swollen, or blistered skin, unusual bruising or bleeding, sore throat, respiratory distress, tightness in the chest, swelling of the mouth, face, lips, or tongue, decreased urination, dark urine, vaginal itching, odor, or discharge, fever, chills, joint pain, and seizures. Prolonged or long-term use of cefadroxil should be avoided. |
|
Precautions |
Cefadroxil should be used only under proper medical health care since it has properties of penicillin allergy, renal function, gastrointestinal tract damage. Pregnant women and breast-feeding women should avoid exposure to cefadroxil. |
InChI:InChI=1/C16H17N3O5S/c1-7-6-25-15-11(14(22)19(15)12(7)16(23)24)18-13(21)10(17)8-2-4-9(20)5-3-8/h2-5,10-11,15,20H,6,17H2,1H3,(H,18,21)(H,23,24)/t10-,11-,15-/m1/s1
50370-12-2 Relevant articles
Efficient synthesis of cefadroxil in [Bmim][NTf2]-phosphate cosolvent by magnetic immobilized penicillin G acylase
Zhaoyu, Zheng,Chunmiao, Hu,Chuanhu, Du,Ping, Xue,Weiwei, Zhang
, p. 1649 - 1657 (2019/11/03)
For the first time, cefadroxil was synth...
PROCESS FOR THE SYNTHESIS OF HYDROXYPHENYLGLYCINE ESTERS
-
Page/Page column 9-10, (2011/10/10)
The present invention refers to a proces...
PROCESS FOR THE CRYSTALLISATION OF CEFADROXIL
-
Page/Page column 5-7, (2008/06/13)
The present invention relates to a proce...
PROCESSES FOR THE PREPARATION OF CEPHEM DERIVATIVES
-
Page/Page column 20, (2008/06/13)
Provided is a process for preparing a co...
50370-12-2 Process route
-
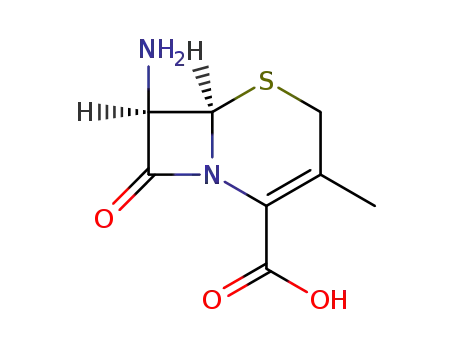
- 22252-43-3,26395-99-3,70287-30-8,72059-35-9
3-deacetyloxy-7-aminocephalosporanic acid

-
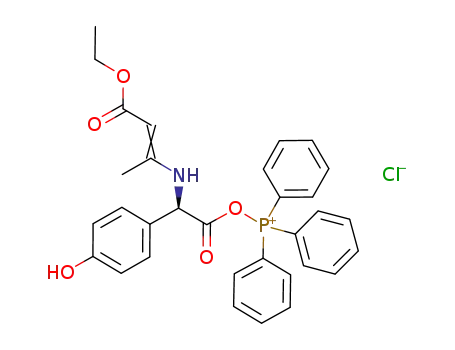
-
((R)-(2-ethoxycarbonyl-1-methyl-vinylamino)-(4-hydroxyphenyl)-acetyloxy)-(triphenyl)phosphonium chloride

-

- 50370-12-2,144790-28-3
cefadroxil
| Conditions | Yield |
|---|---|
|
3-deacetyloxy-7-aminocephalosporanic acid; ((R)-(2-ethoxycarbonyl-1-methyl-vinylamino)-(4-hydroxyphenyl)-acetyloxy)-(triphenyl)phosphonium chloride; With triethylamine; In dichloromethane; water; at -40 - 0 ℃; for 3h;
With hydrogenchloride; In dichloromethane; water; for 1h;
With sodium hydroxide; In dichloromethane; water; at 0 ℃; for 2h; pH=3.2;
|
83% |
-

- 22252-43-3,26395-99-3,70287-30-8,72059-35-9
3-deacetyloxy-7-aminocephalosporanic acid

-
- 78858-51-2
C16H19NO7

-
-
C19H21N3O7S

-

- 50370-12-2,144790-28-3
cefadroxil
| Conditions | Yield |
|---|---|
|
3-deacetyloxy-7-aminocephalosporanic acid; With 1H-imidazole; chloro-trimethyl-silane; In dichloromethane; for 3 - 4h; Heating / reflux;
C16H19NO7; In DMF (N,N-dimethyl-formamide); dichloromethane; at -45 - -20 ℃; for 2.5h;
With hydrogenchloride; In DMF (N,N-dimethyl-formamide); dichloromethane; water; at 0 - 5 ℃; for 0.25h;
|
50370-12-2 Upstream products
-
144790-28-3
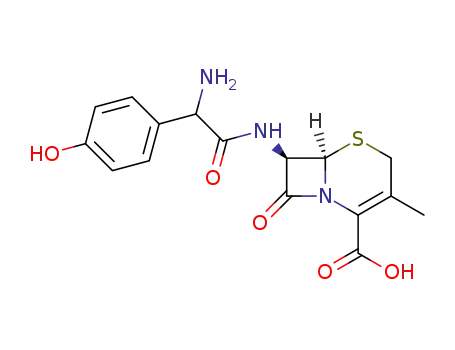
cefadroxil
-
37763-23-8
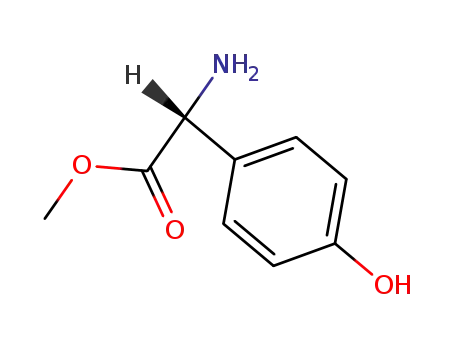
D-2-p-hydroxyphenylglycine methyl ester
-
26395-99-3
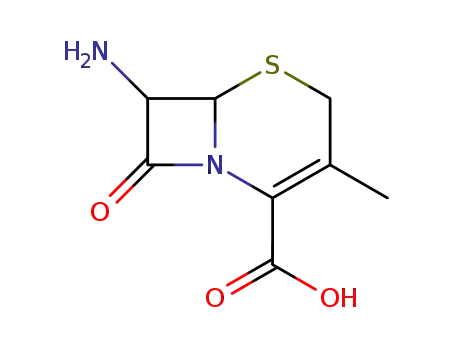
7-aminodesacetoxycephalosporanic acid
-
22252-43-3

3-deacetyloxy-7-aminocephalosporanic acid
50370-12-2 Downstream products
-
73226-86-5
2-hydroxy-3-(p-hydroxyphenyl)-6-methylpyrazine
-
34876-35-2
3-hydroxy-4-methyl-2(5H)-thiophenone
-
147103-95-5
4-hydroxyphenylglycylcefadroxil
Relevant Products
-
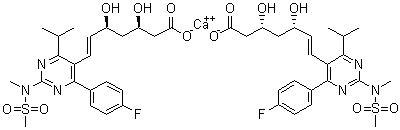
Rosuvastatin calcium
CAS:147098-20-2
-
Cefradine
CAS:38821-53-3
-
Ciprofloxacin Hydrochloride
CAS:86393-32-0