1200-22-2
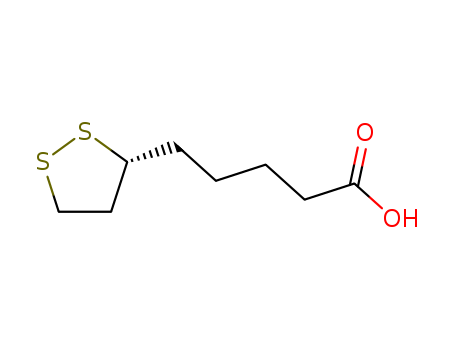
- Product Name:R-(+)-Lipoic acid
- Molecular Formula:C8H14O2S2
- Purity:99%
- Molecular Weight:206.33
Product Details;
CasNo: 1200-22-2
Molecular Formula: C8H14O2S2
Appearance: yellow crystalline solid
Hot Sale Factory Supply High Purity R-(+)-Lipoic acid 1200-22-2 Safe Transportation
- Molecular Formula:C8H14O2S2
- Molecular Weight:206.33
- Appearance/Colour:yellow crystalline solid
- Melting Point:48-52 °C(lit.)
- Refractive Index:114 ° (C=1, EtOH)
- Boiling Point:362.5 °C at 760 mmHg
- PKA:5.4(at 25℃)
- Flash Point:173 °C
- PSA:87.90000
- Density:1.218 g/cm3
- LogP:2.78510
(R)-(+)-1,2-Dithiolane-3-pentanoic acid(Cas 1200-22-2) Usage
|
Chemical Properties |
Yellow Crystalline Solid |
|
Uses |
A fat-metabolism stimulator |
|
General Description |
α-Lipoic acid is an antioxidant which is generally present in plants, animals and many other microorganisms. |
|
Biochem/physiol Actions |
(R)-(+)-α-Lipoic acid was shown to significantly increase pyruvate oxidation while abrogating fatty acid oxidation in rat hepatocytes. These effects make R-(+)-α-Lipoic acid a promising treatment option for the treatment of Type II diabetes. It is a biological antioxidant with prooxidant activities, its therapeutic potential is widely investigated, e.g. in the treatment for Alzheimer′s disease. |
InChI:InChI=1/C8H14O2S2/c9-8(10)4-2-1-3-7-5-6-11-12-7/h7H,1-6H2,(H,9,10)/t7-/m1/s1
1200-22-2 Relevant articles
Enantioselective Synthesis of R-(+)-α-Lipoic Acid
Page, Philip C. Bulman,Rayner, Christopher M.,Sutherland, Ian O.
, p. 1408 - 1409 (1986)
The title compound has been synthesised ...
ASYMMETRIC SYNTHESIS VIA ACETAL TEMPLATES. 12. HIGHLY DIASTEREOSELECTIVE COUPLING REACTIONS WITH A KETENE ACETAL. AN EFFICIENT, ASYMMETRIC SYNTHESIS OF R-(+)-α-LIPOIC ACID.
Elliott, John D.,Steele, John,Johnson, William S.
, p. 2535 - 2538 (1985)
TiCl4 catalyzes the essentially quantita...
Coevolution of the Activity and Thermostability of an ?-Keto Ester Reductase for Better Synthesis of an (R)-α-Lipoic Acid Precursor
Chen, Qi,Xu, Jian-He,Xu, Yao,Zhang, Zhi-Jun,Zheng, Gao-Wei
, (2019)
In this work, we have identified a signi...
Stereocontrolled reactions induced by a thermolabile group. Synthesis of optically active 1,3-diols
Bloch, Robert,Bortolussi, Michel,Girard, Christian,Seck, Matar
, p. 453 - 462 (1992)
Wittig Horner-Michael reactions of phosp...
Microwave-assisted resolution of α-lipoic acid catalyzed by an ionic liquid co-lyophilized lipase
Liu, Ning,Wang, Lei,Wang, Zhi,Jiang, Liyan,Wu, Zhuofu,Yue, Hong,Xie, Xiaona
, p. 9949 - 9960 (2015)
The combination of the ionic liquid co-l...
Preparation method of R-lipoic acid
-
Paragraph 0039; 0065-0067; 0085-0087; 0105-0106, (2021/07/31)
The invention discloses a preparation me...
Synthesis method R -lipoic acid
-
Paragraph 0042; 0070-0074; 0094-0096; 0116-0117, (2021/09/26)
A synthesis method of R -lipoic acid com...
Preparation method of R-lipoic acid tromethamine salt
-
Paragraph 0028; 0036; 0038; 0045; 0047; 0054, (2021/05/12)
The invention relates to a preparation m...
Preparation method of D-lipoic acid
-
Paragraph 0040-0070, (2021/02/24)
The invention relates to a preparation m...
1200-22-2 Process route
-
![(7R,10S)-7-Isopropyl-10-methyl-1,5-dithia-spiro[5.5]undecane](/upload/2023/8/3e5a4b2e-0983-4760-9b56-2f8ad3b10143.png)
- 114529-48-5
(7R,10S)-7-Isopropyl-10-methyl-1,5-dithia-spiro[5.5]undecane

-
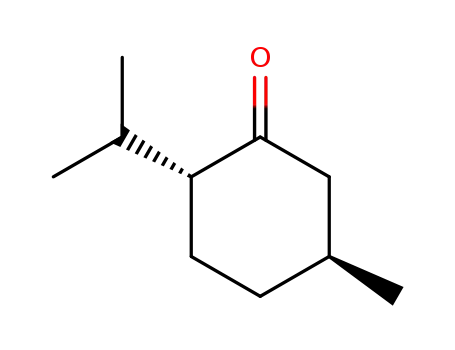
- 3391-87-5,17627-49-5,7786-64-3
l-menthone

-
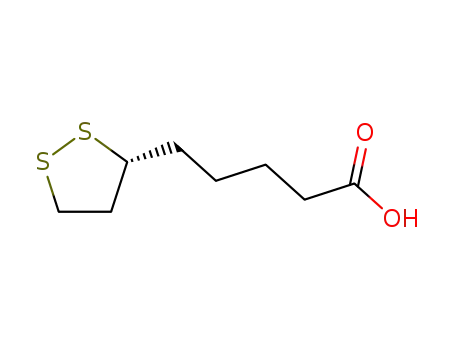
- 1200-22-2
(R)-1,2-dithiolane-3-pentanoic acid
| Conditions | Yield |
|---|---|
|
Multistep reaction;
|
-
![[ethyl (5R)-5-(1,2-dithiolan-3yl)pentanoate]](/upload/2023/8/4da2caf2-6ab9-40a7-a2cf-2293f7f993ec.png)
- 104726-74-1
[ethyl (5R)-5-(1,2-dithiolan-3yl)pentanoate]

-

- 1200-22-2
(R)-1,2-dithiolane-3-pentanoic acid
| Conditions | Yield |
|---|---|
|
With ethanol; water; sodium hydroxide; at 50 ℃; for 2h; Reagent/catalyst; Temperature;
|
96.1% |
|
With sodium hydroxide; In ethanol; water; at 50 ℃; for 2h; Solvent; Temperature;
|
96% |
|
With water; sodium hydroxide; In methanol; at 48 ℃; for 130h; Temperature; Solvent;
|
94% |
|
With hydrogenchloride; potassium hydroxide; In ethanol; for 24h; Ambient temperature;
|
75% |
|
With potassium hydroxide; In ethanol; at 20 ℃; for 24h;
|
75% |
|
With potassium hydroxide; In ethanol; at 20 ℃; for 24h;
|
75% |
|
hydrolysis;
|
|
|
With sodium hydroxide;
|
1200-22-2 Upstream products
-
1077-28-7
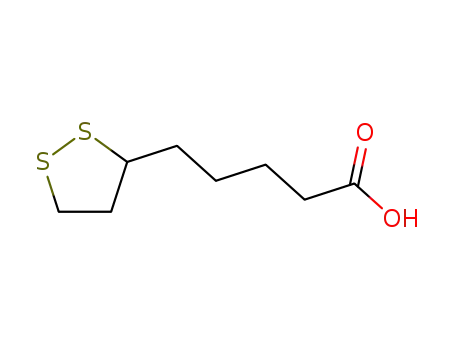
Thioctic acid
-
119365-69-4
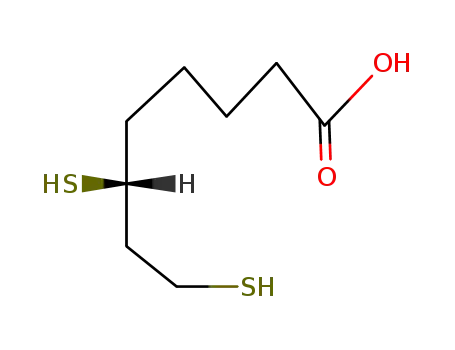
(R)-6,8-dimercaptooctanoic acid
-
107554-84-7
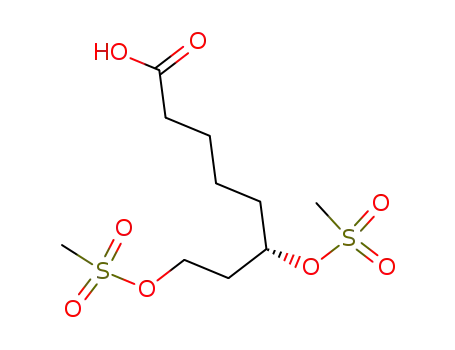
(S)-6,8-dimethylsulfonyloxyoctane-1-carboxylic acid
-
97961-65-4
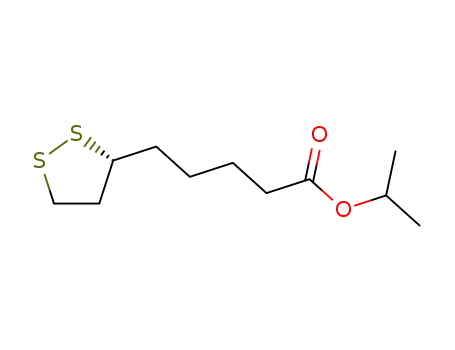
(+)-isopropyl lipoate
1200-22-2 Downstream products
-
108015-78-7
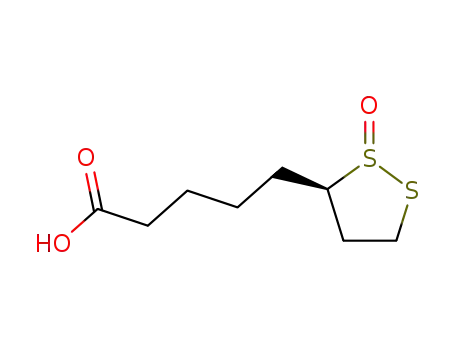
5-(R)-1ξ-oxo-1λ4-[1,2]dithiolan-3-yl-valeric acid
-
119365-69-4

(R)-6,8-dimercaptooctanoic acid
-
462-20-4
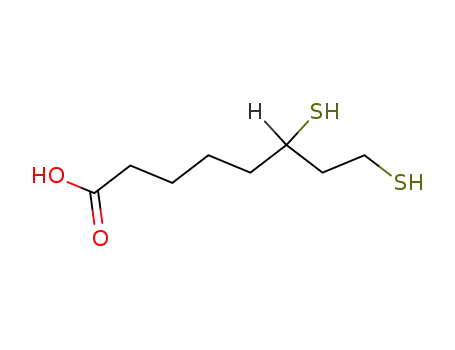
dihydrolipoic acid
Relevant Products
-
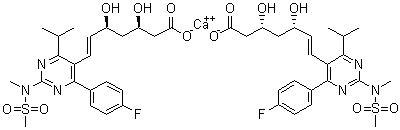
Rosuvastatin calcium
CAS:147098-20-2
-
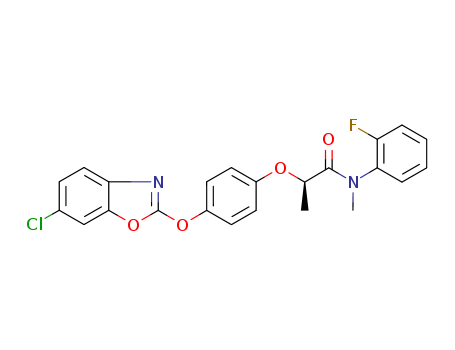
Metamifop
CAS:256412-89-2
-
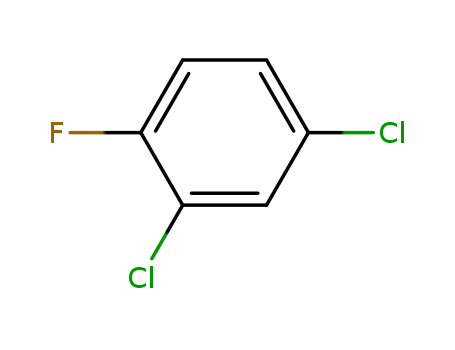
2,4-Dichlorofluorobenzene
CAS:1435-48-9