83121-18-0
- Product Name:Teflubenzuron
- Molecular Formula:C14H6Cl2F4N2O2
- Purity:99%
- Molecular Weight:381.113
Product Details;
CasNo: 83121-18-0
Molecular Formula: C14H6Cl2F4N2O2
Appearance: white crystal
Wholesale Quality Manufacturer Supply Teflubenzuron 83121-18-0 Efficient Delivery
- Molecular Formula:C14H6Cl2F4N2O2
- Molecular Weight:381.113
- Appearance/Colour:white crystal
- Vapor Pressure:8 x 10 -7 mPa (20 °C)
- Melting Point:221-224°
- Refractive Index:1.596
- PKA:8.16±0.46(Predicted)
- PSA:58.20000
- Density:1.646 g/cm3
- LogP:4.97560
Teflubenzuron(Cas 83121-18-0) Usage
|
Chemical properties |
Pure Teflubenzuron is white crystal. m.p.223~225℃ (The original compound 222.5℃),saturated vapor pressure0.8×10-9Pa(20℃), relative density 1.68(20℃).? Its solubility at 20~23℃ are 66g/L in dimethyl sulfoxide, 20g/L in cyclohexanone, 10g/L in acetone, 1.4g/L in ethanol,? 850mg/L in methylbenzene, 50mg/L in hexane,? 0.02mg/L in water. Stable at room temperature. Its hydrolysis half-life period are 5d(pH=7)and 4h (pH=9) at 50℃ and 2~6 weeks in soil. |
|
Uses |
Teflubenzuron’s a fluorobenzoylurea insect growth regulator and a chitinase inhibitor. It achieves the insecticidal purpose through the inhibition of normal molting and development of larvae. It has a particularly high activity on lepidoptera pests, also achieves a good effect when it comes to the larvae of whitefly families, diptera, hymenoptera, coleoptera pests, but it’s invalid for many parasitic insects, predatory insects and spiders. It is mainly used on vegetables, fruit trees, cotton and teabush. E.g. 2000~4000 times diluent of EC can be applied by spraying to larvae of cabbage caterpillar and plutella xylostella in the egg incubation period to 1 to 2 instar.1500~3000 times diluent of EC can be applied by spraying to larvae of plutella xylostella, asparagus caterpillar, prodenia litura in the egg incubation period to 1 to 2 instar, which are resistant to organic phosphorus and pyrethroids. 1500~2000 times diluent of EC can be applied by spraying to cotton bollworm and pink worm in the egg incubation period of the second and third generations, and the elimination rate is about 85% after 10 days. |
|
Production Method |
Method 1 The addition reaction of 2,6-Difluorobenzamide and 2,4-difluoro-3,5-dichlorophenyl isocyanate Method 2 preparation of 2,4-Difluoro-3,5-dichlorophenylamine 2,3,4,5-tetrachloronitrobenzene and Potassium fluoride react in DMF solvent at 140℃ for 15h to obtain 2,4-Difluoro-3,5-dichloronitrobenzene,which is then reduced to 2,4-Difluoro-3,5-dichlorophenylamine Preparation of Teflubenzuron ?As for the preparation of 2,6-difluorobenzoyl isocyanate, see details in the preparation of? Teflubenzuron. ?2,6-difluorobenzoyl isocyanate and 2,4-Difluoro-3,5-dichlorophenylamine react at room temperature for 15h to obtain Teflubenzuron ?As for the nitrification, chlorination, reduction, fluorization and condensation of 1,2,4-Trichlorobenzene to? prepare Teflubenzuron, see details in bibliography. |
|
Hazards & Safety Information |
Category: Pesticide Toxicity Grade: low toxicity Acute Toxicity: LD50: 5000 mg/ kg(Rats oral) Flammability: Flammable with toxic gases of nitrogen oxides, fluorides and chlorides Storage: in dry ventilated storeroom at low temperature, away from food materials Extinguishant: dry powder extinguisher, foam extinguisher, sand. |
|
Metabolic pathway |
There is limited published information on the metabolism of teflubenzuron but an IPCS review is in preparation. Metabolic processes reported are cleavage of the urea bond and hydroxylation of both phenyl rings, including replacement of a fluorine in the 3,5-dichloro-2,4-difluorophenyl ring (see Scheme 1). |
|
Degradation |
Teflubenzuron is more readily hydrolysed at alkaline pH than in acid media. The DT50 at 50 °C was 5 days at pH 7 and 4 hours at pH 9 (PM). |
|
Toxicity evaluation |
Acute oral LD50 for rats: >5,000 mg/kg |
|
Who Evaluation |
Evaluation year: 2015 |
InChI:InChI=1/C14H6Cl2F4N2O2/c15-5-4-8(12(20)10(16)11(5)19)21-14(24)22-13(23)9-6(17)2-1-3-7(9)18/h1-4H,(H2,21,22,23,24)
83121-18-0 Relevant articles
Synthesis method of insecticide teflubenzuron and intermediate 2,6-difluorobenzamide of insecticide teflubenzuron
-
Paragraph 0056; 0062; 0064; 0070; 0072; 0078, (2020/12/10)
The invention discloses a synthesis meth...
Method for exterminating termites
-
, (2008/06/13)
A method for exterminating termites comp...
Method for the preparation of insecticidal benzoylurea compounds
-
, (2008/06/13)
The present invention provides an improv...
Use of acyl urea compounds for controlling endoparasites and ectoparasites of warm-blooded animals
-
, (2008/06/13)
A method of systemically controlling end...
83121-18-0 Process route
-
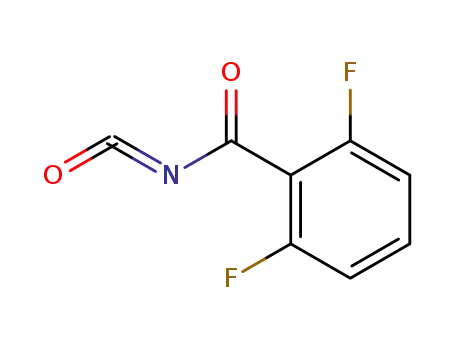
- 60731-73-9
2,6-difluorobenzoyl isocyanate

-
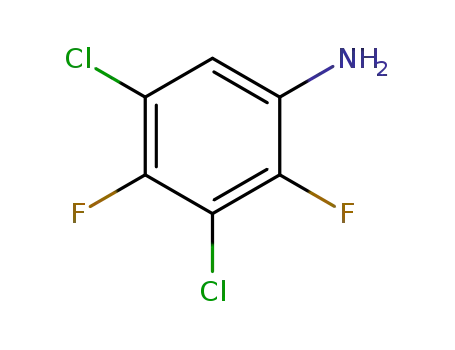
- 83121-15-7
3,5-dichloro-2,4-difluoro-aniline

-
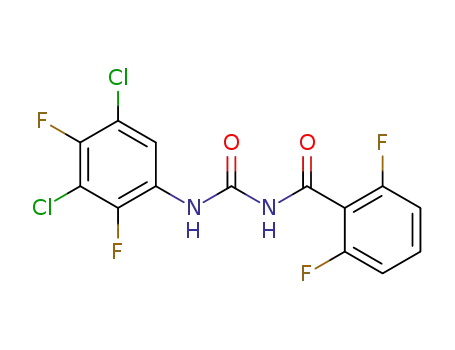
- 83121-18-0,99039-56-2
teflubenzuron
| Conditions | Yield |
|---|---|
|
In chloroform; toluene; for 2h; Reflux;
|
96% |
|
In toluene;
|
|
|
In toluene;
|
-
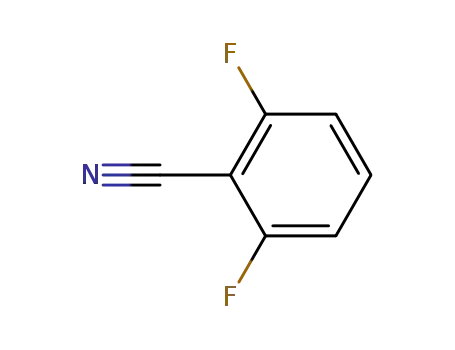
- 1897-52-5
2,6 difluorobenzonitrile

-

- 83121-18-0,99039-56-2
teflubenzuron
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 3 steps
1: dihydrogen peroxide / 6 h / 30 °C
2: chloroform / 4 h / 5 °C / Reflux
3: chloroform; toluene / 2 h / Reflux
With dihydrogen peroxide; In chloroform; toluene;
|
83121-18-0 Upstream products
-
60731-73-9

2,6-difluorobenzoyl isocyanate
-
83121-15-7

3,5-dichloro-2,4-difluoro-aniline
-
1897-52-5

2,6 difluorobenzonitrile
-
118-69-4
2,6-dichlorotoluene
Relevant Products
-
Metamifop
CAS:256412-89-2
-
2,4-Dichlorofluorobenzene
CAS:1435-48-9
-
2,3-Difluoro Phenol
CAS:6418-38-8