129-44-2
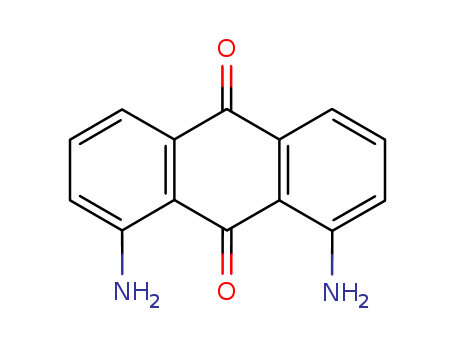
- Product Name:1,5-Diaminoanthraquinone
- Molecular Formula:C14H10 N2 O2
- Purity:99%
- Molecular Weight:238.246
Product Details;
CasNo: 129-44-2
Molecular Formula: C14H10 N2 O2
Appearance: yellow powder
Export Buy Quality 1,5-Diaminoanthraquinone 129-44-2 Lowest Price
- Molecular Formula:C14H10 N2 O2
- Molecular Weight:238.246
- Appearance/Colour:yellow powder
- Vapor Pressure:3.17E-12mmHg at 25°C
- Melting Point:> 300 C
- Refractive Index:1.6500 (estimate)
- Boiling Point:
- PKA:-0.66±0.20(Predicted)
- Flash Point:287.6 ºC
- PSA:86.18000
- Density:1.456 g/cm3
- LogP:2.78880
1,5-Diaminoanthraquinone(Cas 129-44-2) Usage
|
Uses |
1,5-Diaminoanthraquinone (DAAQ) may be used in the preparation of vertical nanowire arrays. It may be used in the synthesis of poly(1,5-diaminoanthraquinone) (PDAA) particles, via chemically oxidative polymerization of DAAQ. |
|
General Description |
1,5-Diaminoanthraquinone (DAAQ) is an organic dye compound. Rapid preparation of 1,5-diaminoanthraquinone nanofibers (DAAQNFs) decorated with small platinum nanoparticles (PtNPs) has been reported. The absorption and fluorescence spectra of DAAQ has been investigated in various organic solvents. Preparation of vertical organic nanowire arrays of DAAQ on solid substrates by a facile physical vapor transport method has been reported. |
|
Purification Methods |
Recrystallise it from aniline (m 313-314o) EtOH or acetic acid [Flom & Barbara J Phys Chem 89 4481 1985]. [Beilstein 14 H 303, 14 I 467, 14 II 116, 14 III 466, 14 IV 479.] |
InChI:InChI=1/C14H10N2O2/c15-9-5-1-3-7-11(9)14(18)8-4-2-6-10(16)12(8)13(7)17/h1-6H,15-16H2
129-44-2 Relevant articles
Vertical organic nanowire arrays: Controlled synthesis and chemical Sensors
Yong, Sheng Zhao,Jinsong, Wu,Jiaxing, Huang
, p. 3158 - 3159 (2009)
-
Quinone Compound-Graphene Composite Material, Preparation Method Thereof, and Flexible Lithium Secondary Battery
-
Paragraph 0075, (2016/11/07)
A quinone compound-graphene composite ma...
A facile method for preparing substituted 1-aminoanthraquinones
Wormser,Sardessai,Abramson
, p. 3211 - 3222 (2007/10/02)
An efficient and simple preparation of α...
SYNTHESIS AND MOLECULAR STRUCTURE OF DERIVATIVES OF 6H-ANTHRA-(9,10-cd)ISOTHIAZOL-6-ONE
Gorelik, M. V.,Alimova, R. A.,Tafeenko, V. A.,Medvedev, S.V.,Shteinman, V. Ya.
, p. 208 - 209 (2007/10/02)
-
129-44-2 Process route
-
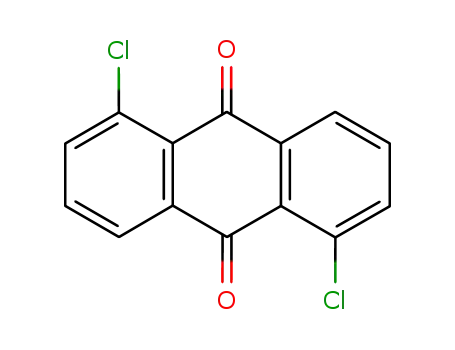
- 82-46-2
1,5-Dichloroanthraquinone

-

- 129-44-2
1,5-diaminoanthraquinone

-
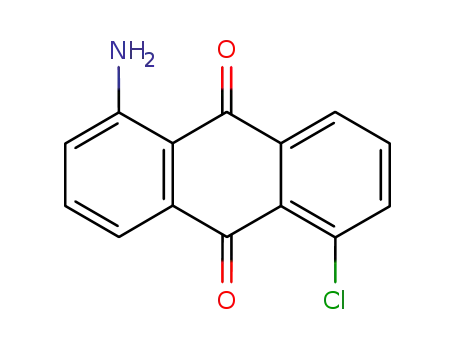
- 117-11-3
1-amino-5-chloroanthracene-9,10-dione
| Conditions | Yield |
|---|---|
|
In ammonia; water;
|
54% |
|
In ammonia; water;
|
53% |
|
In ammonia; water;
|
51.8% |
|
With ammonium hydroxide; at 170 ℃;
|
-
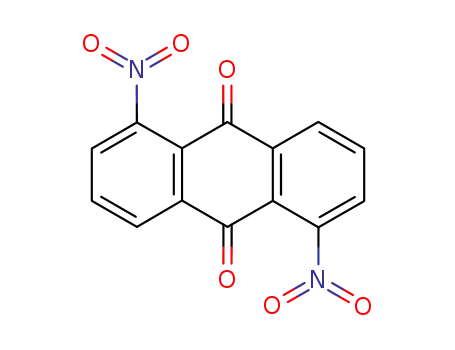
- 82-35-9
1.5-dinitroanthraquinone

-

- 129-44-2
1,5-diaminoanthraquinone
| Conditions | Yield |
|---|---|
|
With acid; durch elektrolytische Reduktion;
|
|
|
With tin oxidepotash;
|
|
|
With phenylhydrazine;
|
|
|
With sodium sulfide;
|
|
|
With copper-magnesium alloy; water;
|
|
|
With sodium dithionite;
|
|
|
With sodium sulfide;
|
|
|
With pyridine; 1,2,3,4-tetrahydro-2-methylquinoline;
|
|
|
With 1,2,3,4-tetrahydro-2-methylquinoline;
|
129-44-2 Upstream products
-
20717-43-5
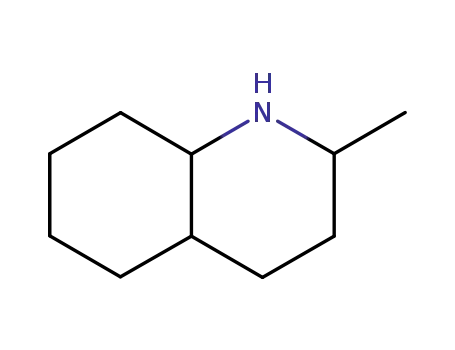
decahydro-2-methylquinoline
-
82-35-9

1.5-dinitroanthraquinone
-
79285-23-7
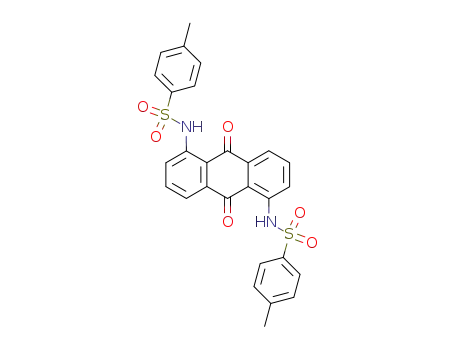
1,5-bis-(toluene-4-sulfonylamino)-anthraquinone
-
853-35-0
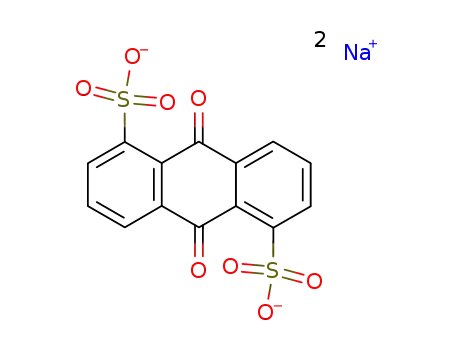
sodium anthraquinone-1,5-disulphonate
129-44-2 Downstream products
-
2987-66-8
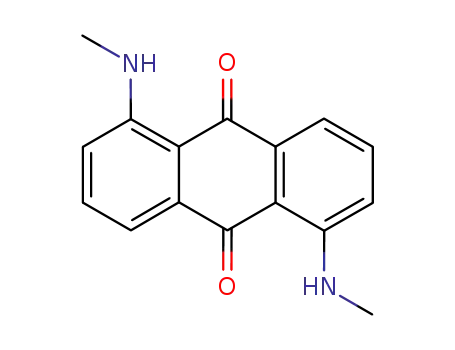
1,5-dimethylaminoanthraquinone
-
871879-82-2
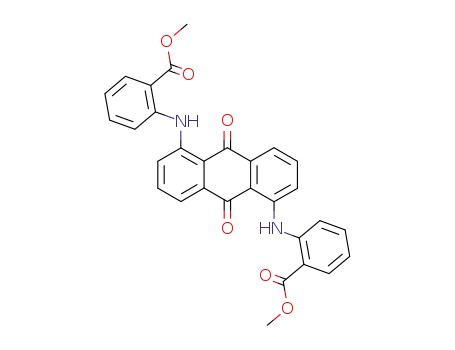
N,N'-(9,10-dioxo-9,10-dihydro-anthracene-1,5-diyl)-di-anthranilic acid dimethyl ester
-
2944-24-3
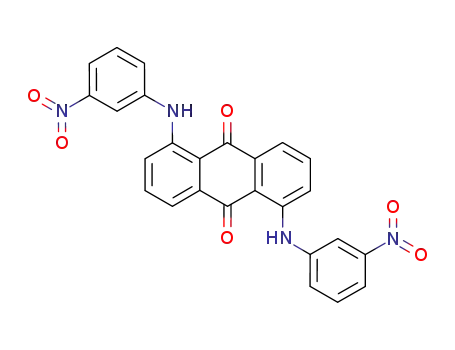
1.5-Bis-(m-nitro-phenylamino)-anthrachinon
-
117-03-3
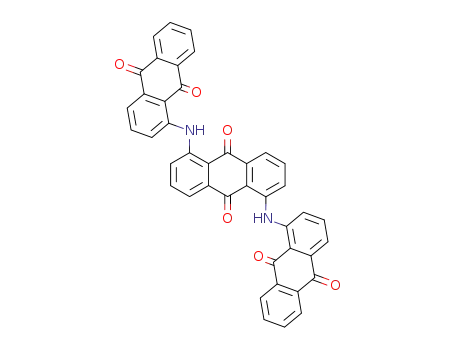
1,5-bis-(9,10-dioxo-9,10-dihydro-[1]anthrylamino)-anthraquinone
Relevant Products
-
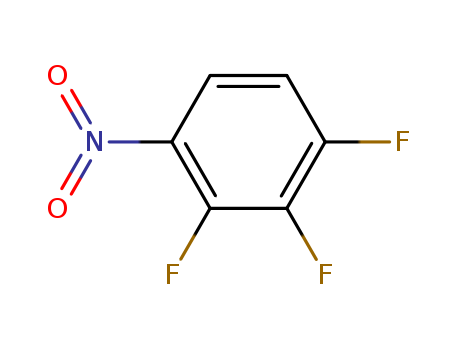
1,2,3-Trifluoro-4-nitrobenzene
CAS:771-69-7
-
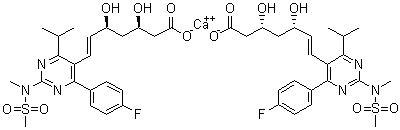
Rosuvastatin calcium
CAS:147098-20-2
-
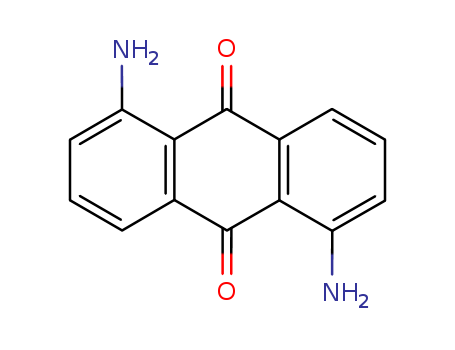
1,8-Diaminoanthraquinone
CAS:129-42-0