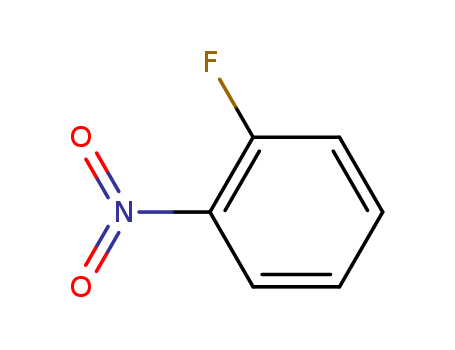
1493-27-2
- Product Name:2-Flouronitrobenzene
- Molecular Formula:C6H4FNO2
- Purity:99%
- Molecular Weight:141.102
Product Details;
CasNo: 1493-27-2
Molecular Formula: C6H4FNO2
Appearance: clear yellow to brownish liquid
Chinese Manufacturer Supply 1493-27-2, Factory Sells 2-Flouronitrobenzene Low Price
- Molecular Formula:C6H4FNO2
- Molecular Weight:141.102
- Appearance/Colour:clear yellow to brownish liquid
- Vapor Pressure:0.000154mmHg at 25°C
- Melting Point:-9 - -6 °C(lit.)
- Refractive Index:n20/D 1.532(lit.)
- Boiling Point:228.863 °C at 760 mmHg
- Flash Point:94.444 °C
- PSA:45.82000
- Density:1.338 g/cm3
- LogP:2.25710
1-Fluoro-2-nitrobenzene(Cas 1493-27-2) Usage
|
Chemical Properties |
clear yellow to brownish liqui |
|
Uses |
o-Nitrofluorobenzene is an intermediate in the synthetic preparations of various pharmaceutical goods. |
|
Synthesis Reference(s) |
Tetrahedron, 52, p. 23, 1996 DOI: 10.1016/0040-4020(95)00867-8Tetrahedron Letters, 26, p. 2233, 1985 DOI: 10.1016/S0040-4039(00)98970-6 |
InChI:InChI=1/C7H8BFO3/c1-12-7-3-2-5(8(10)11)4-6(7)9/h2-4,10-11H,1H3
1493-27-2 Relevant articles
Method for synthesizing nitro (hetero) aromatic hydrocarbon
-
Paragraph 0082-0084; 0097-0099, (2022/04/08)
The invention discloses a method for syn...
Nitration of aromatics with dinitrogen pentoxide in a liquefied 1,1,1,2-tetrafluoroethane medium
Fauziev, Ruslan V.,Kharchenko, Alexandr K.,Kuchurov, Ilya V.,Zharkov, Mikhail N.,Zlotin, Sergei G.
, p. 25841 - 25847 (2021/08/09)
Regardless of the sustainable developmen...
Ipso Nitration of Aryl Boronic Acids Using Fuming Nitric Acid
Baucom, Kyle D.,Brown, Derek B.,Caille, Seb,Murray, James I.,Quasdorf, Kyle,Silva Elipe, Maria V.
supporting information, (2021/06/30)
The ipso nitration of aryl boronic acid ...
Radical Decarboxylative Carbometalation of Benzoic Acids: A Solution to Aromatic Decarboxylative Fluorination
Xu, Peng,López-Rojas, Priscila,Ritter, Tobias
supporting information, p. 5349 - 5354 (2021/05/05)
Abundant aromatic carboxylic acids exist...
1493-27-2 Process route
-
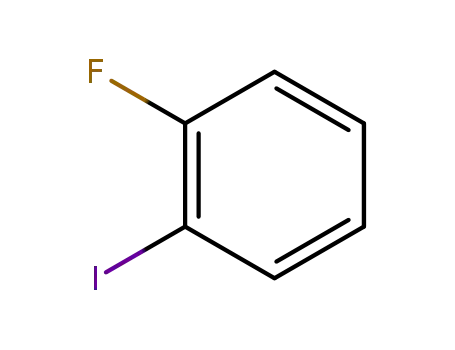
- 348-52-7
1-Fluoro-2-iodobenzene

-
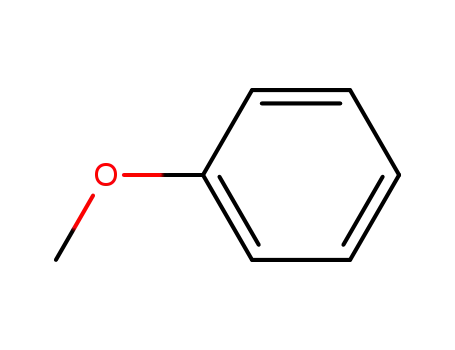
- 100-66-3
methoxybenzene

-
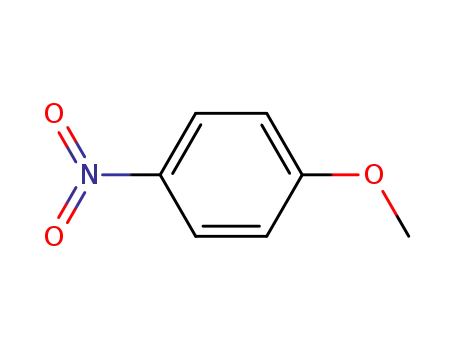
- 100-17-4
para-methoxynitrobenzene

-
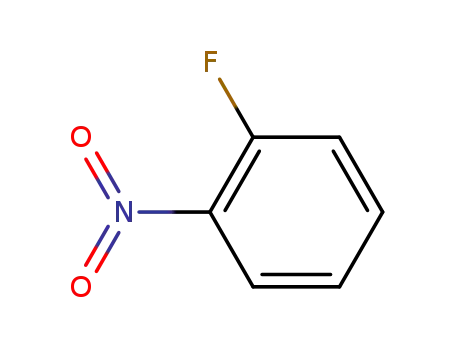
- 1493-27-2,127723-77-7
ortho-nitrofluorobenzene
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 2 steps
1: 3-chloro-benzenecarboperoxoic acid / 16 h / 20 °C / Sealed tube
2: sodium nitrite; sodium hydrogencarbonate / dichloromethane; water / 3 h / 60 °C / Sealed tube
With sodium hydrogencarbonate; 3-chloro-benzenecarboperoxoic acid; sodium nitrite; In dichloromethane; water;
|
-
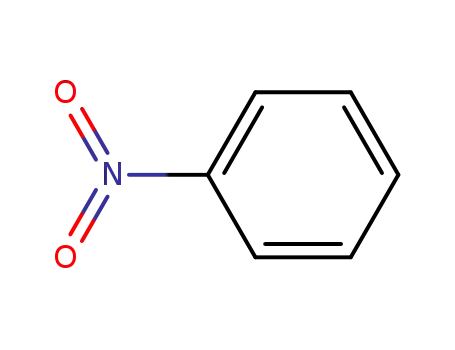
- 98-95-3,26969-40-4
nitrobenzene

-
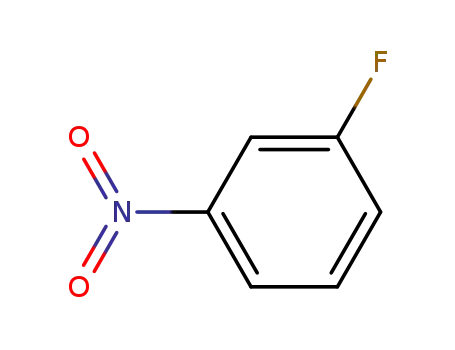
- 402-67-5
3-fluoro-1-nitrobenzene

-
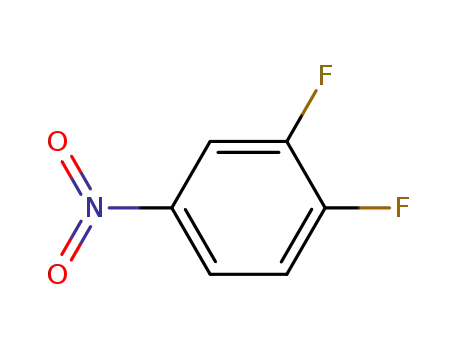
- 369-34-6
3,4-difluoronitrobenzene

-
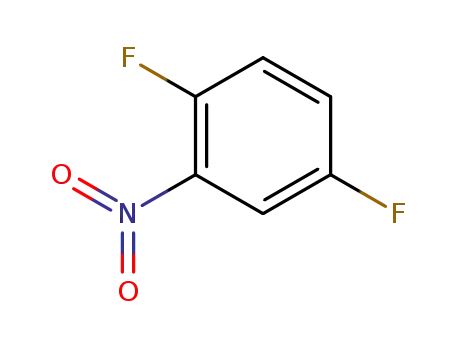
- 364-74-9
1,4-difluoro-2-nitrobenzene

-
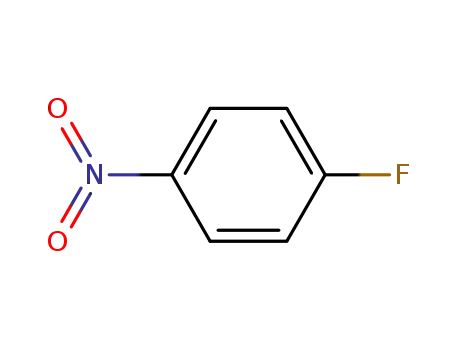
- 350-46-9,178603-76-4
4-Fluoronitrobenzene

-

- 1493-27-2,127723-77-7
ortho-nitrofluorobenzene
| Conditions | Yield |
|---|---|
|
With xenon difluoride; boron trifluoride diethyl etherate; at 0 - 25 ℃; Reagent/catalyst; Cooling with ice;
|
0.05 mmol 0.16 mmol 0.01 mmol 0.016 mmol 0.001 mmol |
1493-27-2 Upstream products
-
462-06-6
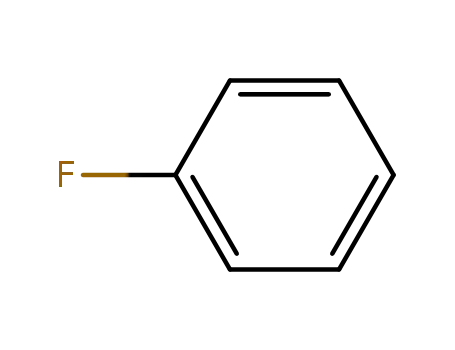
fluorobenzene
-
591-09-3
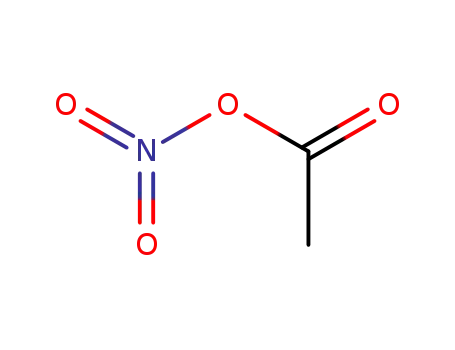
nitro acetate
-
364-76-1
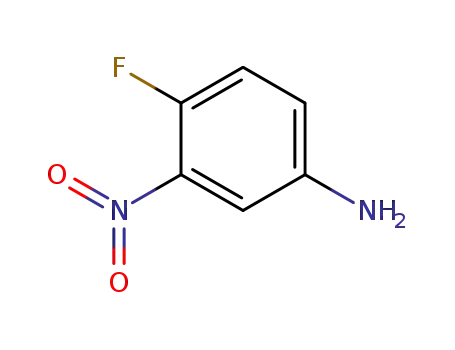
4-Fluoro-3-nitroaniline
-
88-73-3
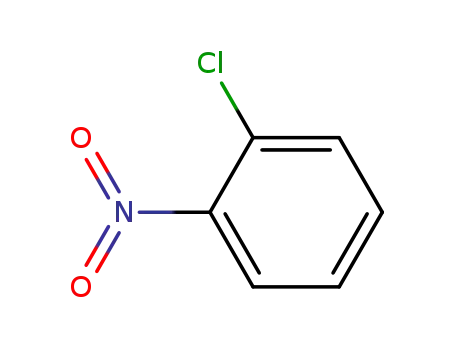
2-Chloronitrobenzene
1493-27-2 Downstream products
-
6373-71-3
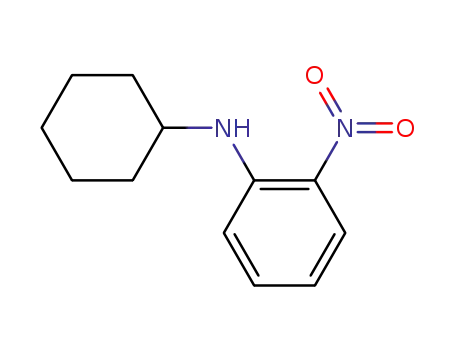
N-cyclohexyl-2-nitroaniline
-
52753-44-3
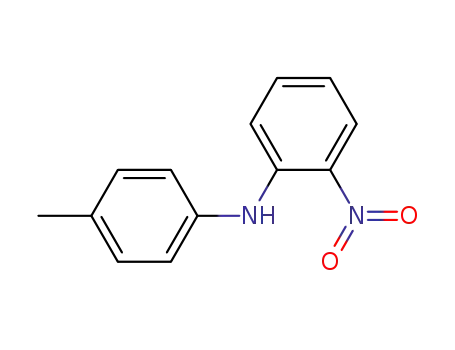
(2-nitrophenyl)-p-tolylamine
-
54381-13-4
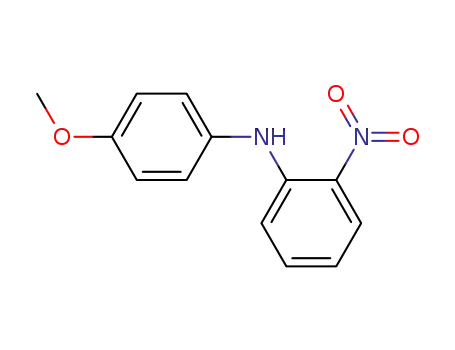
2-nitro-4'-methoxydiphenylamine
-
10112-15-9
N-Ethyl-2-nitroaniline
Relevant Products
-
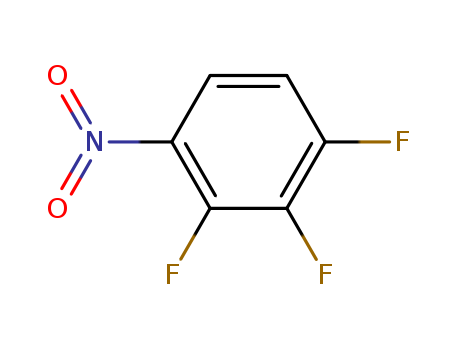
1,2,3-Trifluoro-4-nitrobenzene
CAS:771-69-7
-
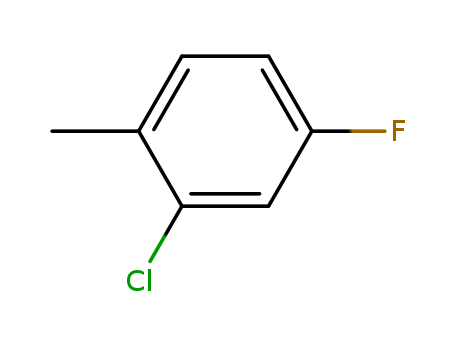
2-Chloro-4-fluorotoluene
CAS:452-73-3
-
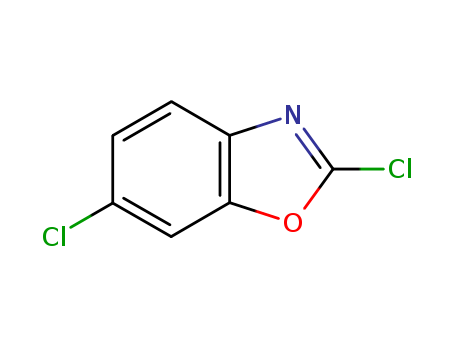
2,6-Dichlorobenzoxazole
CAS:3621-82-7