608-25-3
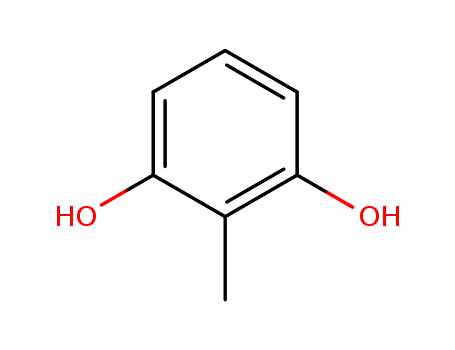
- Product Name:2-METHYLRESORCINOL
- Molecular Formula:C7H8O2
- Purity:99%
- Molecular Weight:124.139
Product Details;
CasNo: 608-25-3
Molecular Formula: C7H8O2
Appearance: White to off-white crystal powder
Hot Sale Quality Factory Supply 2-METHYLRESORCINOL 608-25-3 Cheapest Price
- Molecular Formula:C7H8O2
- Molecular Weight:124.139
- Appearance/Colour:White to off-white crystal powder
- Vapor Pressure:0.00201mmHg at 25°C
- Melting Point:114-120 °C(lit.)
- Refractive Index:1.594
- Boiling Point:282.1 °C at 760 mmHg
- PKA:pK1:10.05;pK2:11.64 (25°C,μ=0.65)
- Flash Point:142.9 °C
- PSA:40.46000
- Density:1.21 g/cm3
- LogP:1.40620
2-Methylresorcinol(Cas 608-25-3) Usage
|
Chemical Properties |
White to off-white crystal powder |
|
Uses |
2-Methylresorcinol is used in the preparation of aromatic benziporphyrins, tripyrrane analogs and C-5-bromo-2-hydroxyphenylcalix[4]-2-methylresorcinarene. Further, it is employed in oxidative and non-oxidative hair dye formulations. |
|
Application |
2-Methylresorcinol is used as medicine, pesticide, dye intermediate, hair auxiliaries, etc. |
|
Preparation |
Synthesis of 2-methylresorcinol: The synthetic routes to an alkylresorcinol that is partially alkylated at a position other than the 4th position on the aromatic ring are multistep and give low overall yields. For example, a 50% yield of a 2-methylresorcinol is obtained by hydrogenation of resorcinol and methylation of the resulting dihydroresorcinol to 2-methylcyclohexane-1,3-dione which on treatment with bromine is converted into 4,6-dibromo-2-methylresorcinol and finally hydrogenolysis of the dibromo derivative to produce the 2-methylresorcinol. Another example is that 5-methylresorcinol can be prepared by a five-step synthesis starting with p-toluidine or a four-step synthesis starting from ethyl crotonate. The five-step synthesis involves acetylation, two-step nitration, reductive hydrogenation, and hydrolysis.Literature source US04086281 |
|
General Description |
The reaction between 2-methylresorcinol and 2-alkenals was studied to investigate the scavenging ability of m-diphenols for the 2-alkenals formed during lipid oxidation. |
|
Flammability and Explosibility |
Nonflammable |
InChI:InChI=1/C7H8O2/c1-5-6(8)3-2-4-7(5)9/h2-4,8-9H,1H3
608-25-3 Relevant articles
Method for synthesizing M-hydroxyanisole
-
Paragraph 0032-0033; 0052-0053; 0056-0057; 0060-0063, (2021/11/03)
The invention provides a method for synt...
Method for simultaneously preparing 2-methyl resorcinol and 4-methyl resorcinol
-
Paragraph 0034-0066, (2022/01/10)
The invention provides a method for simu...
Synthesis method of 2,6-dihydroxytoluene
-
Paragraph 0047-0049, (2021/11/27)
The invention belongs to the technical f...
Method for continuously producing 2, 6-dihydroxytoluene
-
, (2021/08/11)
The invention discloses a method for con...
608-25-3 Process route
-
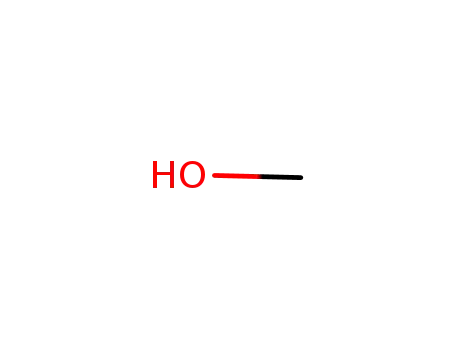
- 67-56-1
methanol

-
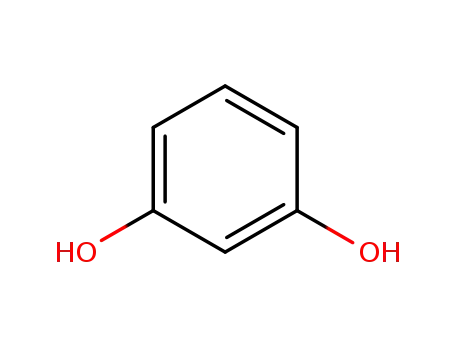
- 108-46-3
recorcinol

-
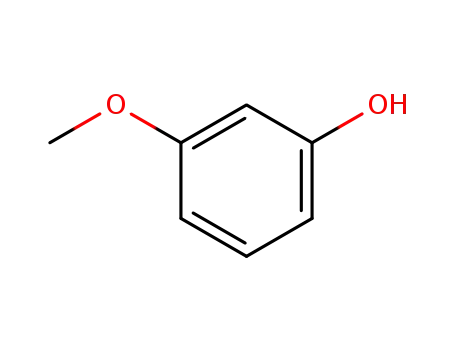
- 150-19-6
O-methylresorcine

-
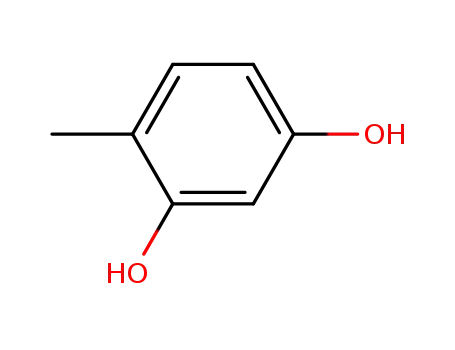
- 496-73-1,73073-80-0
4-methyl resorcinol

-
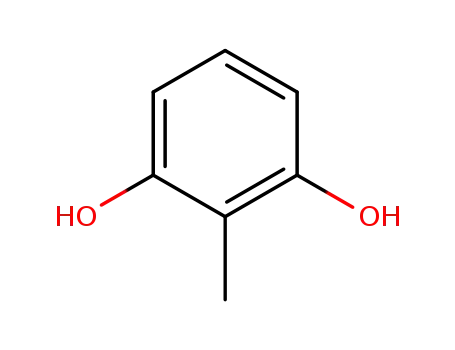
- 608-25-3
2-methylbenzene-1,3-diol

-
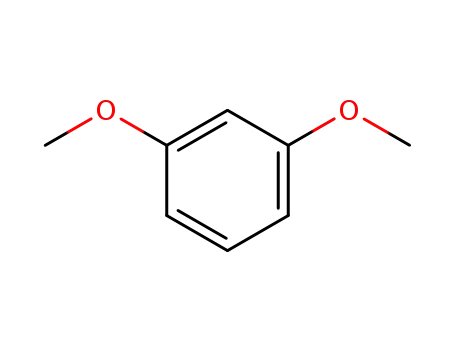
- 151-10-0
1,3-Dimethoxybenzene
| Conditions | Yield |
|---|---|
|
With lanthanum phosphate-alumina; at 290 ℃; Temperature; Reagent/catalyst; Inert atmosphere;
|
-
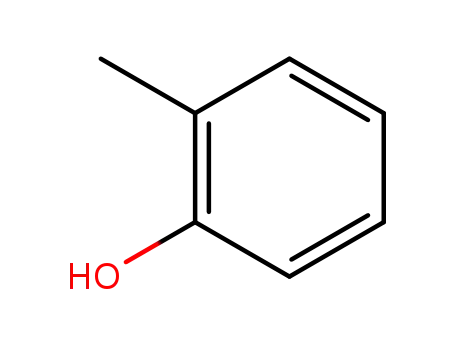
- 95-48-7,77504-84-8
ortho-cresol

-

- 496-73-1,73073-80-0
4-methyl resorcinol

-
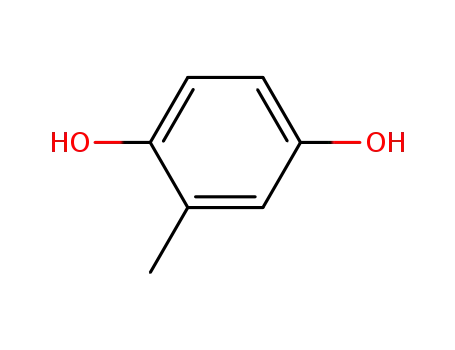
- 95-71-6,96937-50-7
2-methylbenzene-1,4-diol

-

- 608-25-3
2-methylbenzene-1,3-diol

-
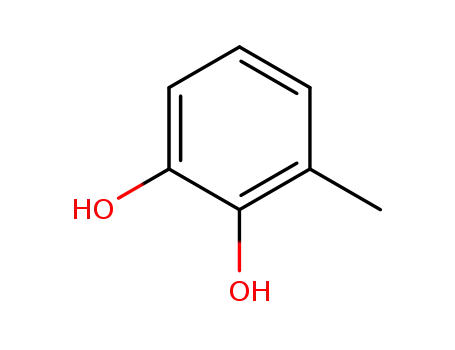
- 488-17-5
3-methylbenzene-1,2-diol
| Conditions | Yield |
|---|---|
|
With hydrogen fluoride; dihydrogen peroxide; antimony pentafluoride; at -40 ℃; for 0.5h; Product distribution;
|
17% 4% 3% 3% |
|
With hydrogen fluoride; dihydrogen peroxide; antimony pentafluoride; at -40 ℃; for 0.5h;
|
17% 3% 3% 4% |
608-25-3 Upstream products
-
6971-52-4
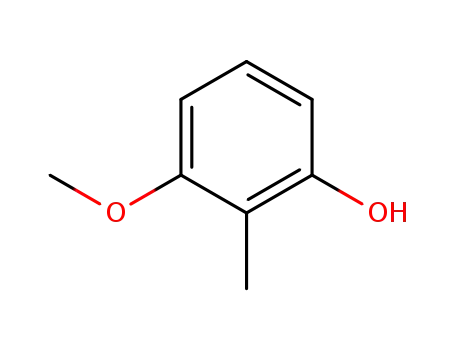
3-methoxy-2-methylphenol
-
387-46-2
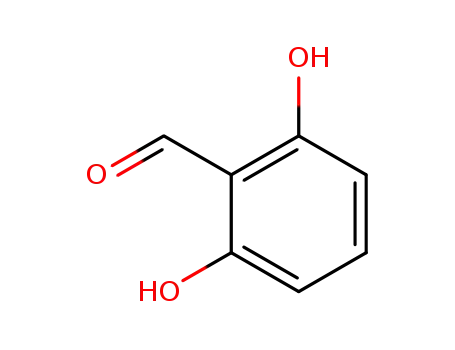
2,6-dihydroxybenzaldehyde
-
4707-49-7
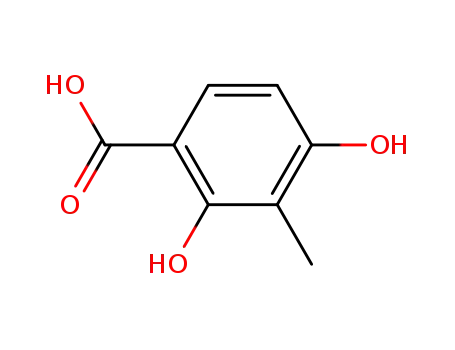
2,4-dihydroxy-3-methylbenzoic acid
-
33662-58-7
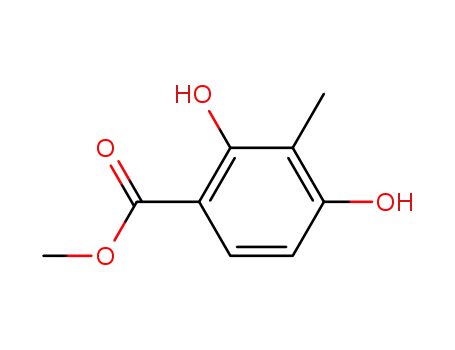
methyl 2,4-dihydroxy-3-methylbenzoate
608-25-3 Downstream products
-
109342-74-7
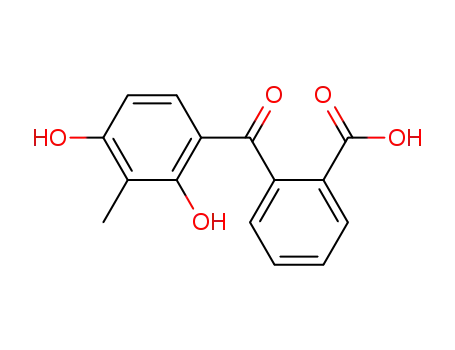
2-(2,4-dihydroxy-3-methyl-benzoyl)-benzoic acid
-
118797-71-0
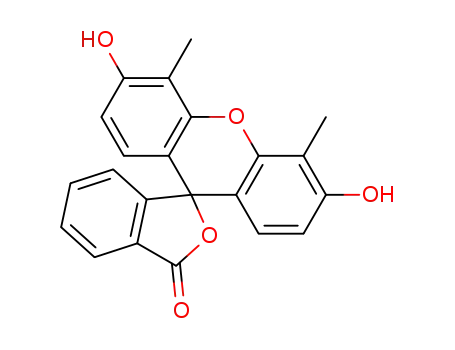
3′,6′?dihydroxy?4′,5′?dimethyl?3H?spiro[2?benzofuran?1,9′?xanthen]?3?one
-
93970-93-5
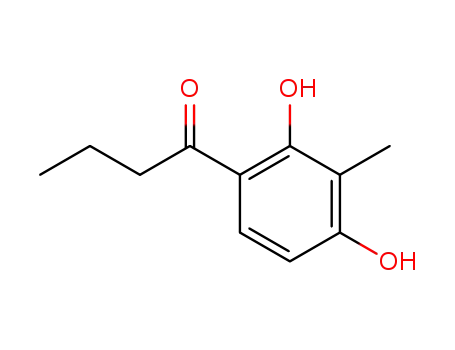
2,4-dihydroxy-3-methylbutyrophenone
-
2732-17-4
7-hydroxy-8-methyl-2H-chromen-2-one
Relevant Products
-
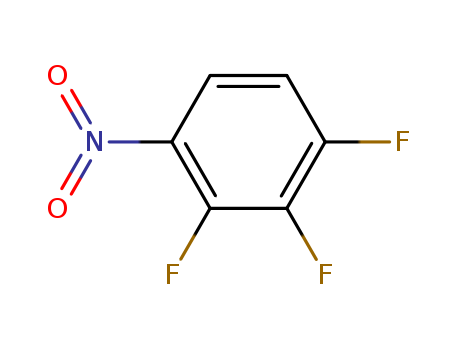
1,2,3-Trifluoro-4-nitrobenzene
CAS:771-69-7
-
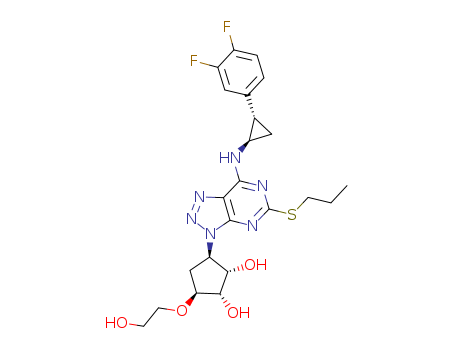
Ticargrelor
CAS:274693-27-5
-
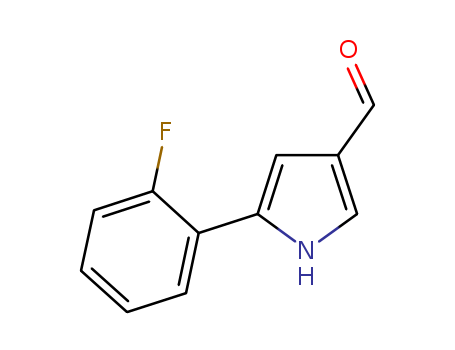
5-(2-fluorophenyl)-1H-Pyrrole-3-carboxaldehyde
CAS:881674-56-2