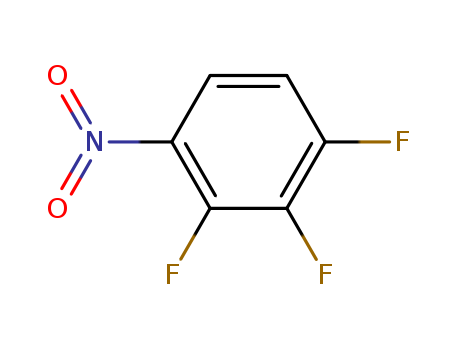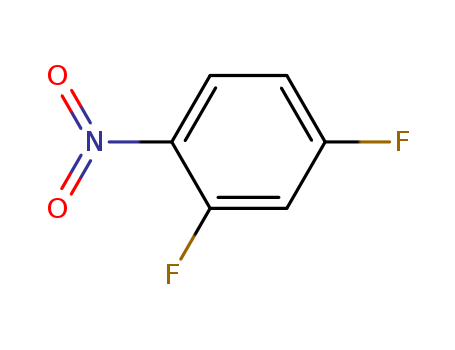
446-35-5
- Product Name:2,4-Difluoronitrobenzene
- Molecular Formula:C6H3F2NO2
- Purity:99%
- Molecular Weight:159.092
Product Details;
CasNo: 446-35-5
Molecular Formula: C6H3F2NO2
Appearance: yellow liquid
Top Purity 99% Buy Quality 2,4-Difluoronitrobenzene 446-35-5 Cheap Price
- Molecular Formula:C6H3F2NO2
- Molecular Weight:159.092
- Appearance/Colour:yellow liquid
- Vapor Pressure:0.331mmHg at 25°C
- Melting Point:9-10 °C(lit.)
- Refractive Index:n20/D 1.511(lit.)
- Boiling Point:207 °C at 760 mmHg
- Flash Point:90.6 °C
- PSA:45.82000
- Density:1.45 g/cm3
- LogP:2.39620
2,4-Difluoronitrobenzene(Cas 446-35-5) Usage
|
Synthesis |
KF with n-Hexadecyltrimethylammonium bromide as the PTC has been shown recently to work in dimethylformamide (DMF), important owing to the low cost16 of DMF. |
|
Chemical Properties |
yellow liquid |
|
Uses |
2,4-Difluoronitrobenzene has been used in the synthesis of: 2,4-difluoro-5-nitrobenzenesulfonic acid via sulfonation reaction; (±)-horsfiline; resin-bound 2-arylaminobenzimidazoles.2,?4-?Difluoronitrobenzene is a reactant in the preparation of 4-?thiazolidinone derivatives as antimicrobial agents. |
|
General Description |
Nucleophilic aromatic substitution of 2,4-difluoronitrobenzene with morpholine has been investigated using flow reactor with simulated moving bed (SMB) chromatography module. |
446-35-5 Relevant articles
Inexpensive, Active KF for Nucleophilic Aromatic Displacement Reactions
Smyth, Timothy P.,Carey, Aedin,Hodnett, B. K.
, p. 6363 - 6376 (1995)
The simple, and inexpensive process, of ...
Novel preparation method of 2, 4, 5-trifluorophenylacetic acid
-
, (2021/06/23)
The invention discloses a novel preparat...
Preparation method of fluorine-containing aryl compound
-
Paragraph 0126-0134, (2021/06/12)
The invention relates to the field of or...
Efficient synthesis method of meta-fluoranisole (by machine translation)
-
, (2020/06/05)
The method is characterized by comprisin...
Novel manufacturing method of fluoro-aryl compounds and derivatives thereof
-
Paragraph 0201-0205; 0219-0224, (2020/01/25)
The invention relates to a novel method ...
446-35-5 Process route
-
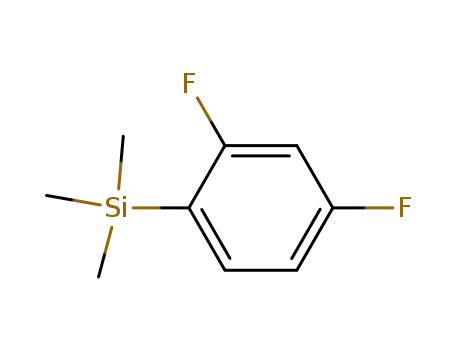
- 148854-10-8
(2,4-difluorophenyl)trimethylsilane

-
- 372-18-9
1,3-Difluorobenzene

-
- 446-35-5
2,4-Difluoronitrobenzene

-

- 367-82-8
3-Fluoro-4,6-dinitrophenol
| Conditions | Yield |
|---|---|
|
With water; nitric acid; acetic anhydride; at 45 ℃; for 22h;
|
2.3% 32.7% 6.8% |
-

- 133117-48-3
1,3-difluoro-2-trimethylsilylbenzene

-

- 372-18-9
1,3-Difluorobenzene

-

- 19064-24-5
2,6-difluoro-1-nitrobenzene

-

- 446-35-5
2,4-Difluoronitrobenzene

-

- 186315-85-5
1,3-difluoro-4-nitro-2-(trimethylsilyl)benzene
| Conditions | Yield |
|---|---|
|
With water; nitric acid; acetic anhydride; at 65 ℃; for 24h;
|
13.4% 11.7% 2.6% |
446-35-5 Upstream products
-
611-06-3

2,4-dichloronitrobenzene
-
98-95-3

nitrobenzene
-
2369-13-3
3-fluoro-4-nitroaniline
-
148854-10-8
(2,4-difluorophenyl)trimethylsilane
446-35-5 Downstream products
-
53013-41-5
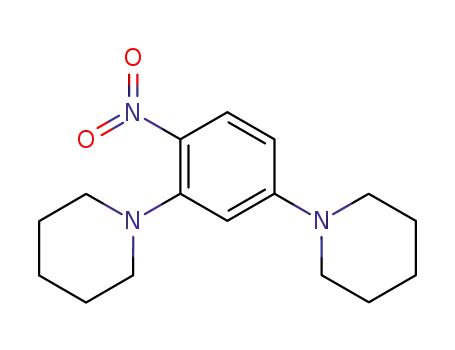
4-(2-nitro-5-piperidinophenyl)piperidine
-
448-19-1
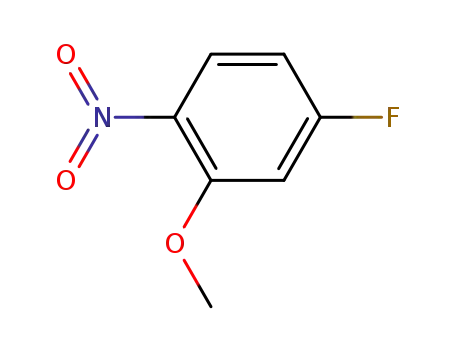
4-fluoro-2-methoxy-1-nitrobenzene
-
4920-84-7
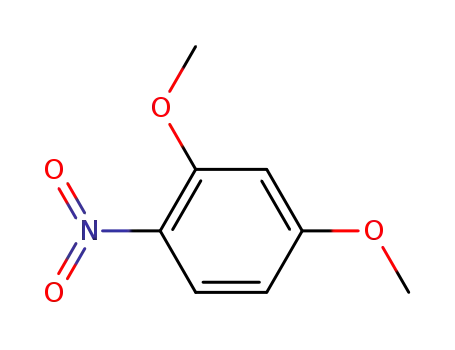
2,4-dimethoxynitrobenzene
-
327-92-4

1,5-difluoro-2,4-dinitrobenzene
Relevant Products
-
1,2,3-Trifluoro-4-nitrobenzene
CAS:771-69-7
-
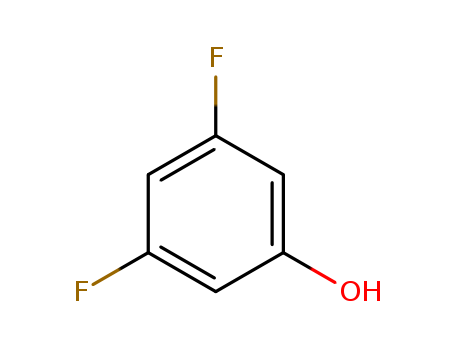
3,5-Difluorophenol
CAS:2713-34-0
-
Atorvastatin Acetonide tert-Butyl Ester
CAS:125971-95-1