125971-95-1
- Product Name:Atorvastatin Acetonide tert-Butyl Ester
- Molecular Formula:C40H47FN2O5
- Purity:99%
- Molecular Weight:654.822
Product Details;
CasNo: 125971-95-1
Molecular Formula: C40H47FN2O5
Top Purity 99% Buy High Quality Atorvastatin Acetonide tert-Butyl Ester 125971-95-1 Safe Shipping
- Molecular Formula:C40H47FN2O5
- Molecular Weight:654.822
- Melting Point:144-148 °C
- Boiling Point:678.035 °C at 760 mmHg
- PKA:13.57±0.70(Predicted)
- Flash Point:363.863 °C
- PSA:78.79000
- Density:1.15 g/cm3
- LogP:9.44200
tert-Butyl (4R,6R)-2-[[[6-(2-4-fluorophenyl)-5-isopropyl-3-phenyl-4-(phenylcarbamoyl)pyrrol-1-yl]ethyl]-2,2-dimethyl-1,3-dioxan-4-yl]acetate(Cas 125971-95-1) Usage
|
Chemical Properties |
White Solid |
|
Uses |
Atorvastatin intermediate |
InChI:InChI:1S/C40H47FN2O5/c1-26(2)36-34(27-14-10-8-11-15-27)35(38(45)42-30-16-12-9-13-17-30)37(28-18-20-29(41)21-19-28)43(36)23-22-31-24-32(47-40(6,7)46-31)25-33(44)48-39(3,4)5/h8-21,26,31-32H,22-25H2,1-7H3,(H,42,45)
125971-95-1 Relevant articles
AN IMPROVED AND COMMERCIALLY VIABLE PROCESS FOR PREPARATION OF PYRROLE DERIVATIVES WITH IMPROVED IMPURITY PROFILE & MINIMISATION OF UNIT OPERATIONS.
-
Page/Page column 68-69; 71, (2020/02/14)
The present invention relates to improve...
[18F]Atorvastatin: synthesis of a potential molecular imaging tool for the assessment of statin-related mechanisms of action
Antunes, Inês F.,Clemente, Gon?alo S.,D?mling, Alexander,Elsinga, Philip H.,Rickmeier, Jens,Ritter, Tobias,Zarganes-Tzitzikas, Tryfon
, (2020/04/24)
Background: Statins are lipid-lowering a...
Novel method for preparing atorvastatin key intermediate L1 through solvent-free method
-
Paragraph 0037-0086, (2019/11/12)
The invention discloses a novel method f...
Separating method for impurity A and impurity B and method for effectively reducing content of impurity A in atorvastatin calcium condensate
-
Paragraph 0071; 0072; 0073, (2019/01/16)
The invention discloses a separating met...
125971-95-1 Process route
-
- 1331869-19-2
C26H22FNO3

-
![tert-butyl [(4R,6R)-6-aminoethyl-2,2-dimethyl-1,3-dioxan-4-yl]acetate](/upload/2023/8/7646f4c7-c9b7-440f-a99b-c74fc56fab3d.png)
- 125971-86-0,125995-13-3
tert-butyl [(4R,6R)-6-aminoethyl-2,2-dimethyl-1,3-dioxan-4-yl]acetate

-
![tert-butyl (4R,6R)-6-{2-[2-(4-fluorophenyl)-5-isopropyl-3-phenyl-4-(phenylcarbamoyl)pyrrol-1-yl]ethyl}-2,2-dimethyl-1,3-dioxane-4-acetate](/upload/2023/8/a0859b7c-79a0-4166-8fae-0cec278c0b17.png)
- 125971-95-1
tert-butyl (4R,6R)-6-{2-[2-(4-fluorophenyl)-5-isopropyl-3-phenyl-4-(phenylcarbamoyl)pyrrol-1-yl]ethyl}-2,2-dimethyl-1,3-dioxane-4-acetate
| Conditions | Yield |
|---|---|
|
With 1,8-diazabicyclo[5.4.0]undec-7-ene; Trimethylacetic acid; In cyclohexane; at 80 - 84 ℃; for 25h; Concentration; Reagent/catalyst; Dean-Stark; Reflux;
|
79% |
|
With Trimethylacetic acid; In tetrahydrofuran; hexane; toluene; at 110 ℃; for 30h; Inert atmosphere;
|
-
![tert-butyl [(4R,6R)-6-aminoethyl-2,2-dimethyl-1,3-dioxan-4-yl]acetate](/upload/2023/8/7646f4c7-c9b7-440f-a99b-c74fc56fab3d.png)
- 125971-86-0,125995-13-3
tert-butyl [(4R,6R)-6-aminoethyl-2,2-dimethyl-1,3-dioxan-4-yl]acetate

-
![2-[2-(4-fluorophenyl)-2-oxo-1-phenylethyl]-4-methyl-3-oxopentanoic acid phenylamide](/upload/2023/8/6f668e00-cac5-4d1a-a4be-57cb5f5e098d.png)
- 125971-96-2,125971-58-6
2-[2-(4-fluorophenyl)-2-oxo-1-phenylethyl]-4-methyl-3-oxopentanoic acid phenylamide

-
![tert-butyl (4R,6R)-6-{2-[2-(4-fluorophenyl)-5-isopropyl-3-phenyl-4-(phenylcarbamoyl)pyrrol-1-yl]ethyl}-2,2-dimethyl-1,3-dioxane-4-acetate](/upload/2023/8/a0859b7c-79a0-4166-8fae-0cec278c0b17.png)
- 125971-95-1
tert-butyl (4R,6R)-6-{2-[2-(4-fluorophenyl)-5-isopropyl-3-phenyl-4-(phenylcarbamoyl)pyrrol-1-yl]ethyl}-2,2-dimethyl-1,3-dioxane-4-acetate
| Conditions | Yield |
|---|---|
|
With Trimethylacetic acid; In toluene; at 105 - 110 ℃; for 1h; Industrial scale;
|
96.5% |
|
With tetra(n-butyl)ammonium hydrogensulfate; diisopropylamine; Trimethylacetic acid; at 78 - 85 ℃; for 40h;
|
82.3% |
|
With Trimethylacetic acid; In tetrahydrofuran; n-heptane; toluene; Heating;
|
75% |
|
With Trimethylacetic acid; In tetrahydrofuran; for 40 - 72h; Heating / reflux;
|
75.1% |
|
With Trimethylacetic acid; In tetrahydrofuran; n-heptane; toluene; for 48h; Heating / reflux;
|
72.3% |
|
With tetra(n-butyl)ammonium hydrogensulfate; Trimethylacetic acid; at 120 ℃; for 5.5h; Temperature; Reagent/catalyst;
|
69.6% |
|
With Trimethylacetic acid; In tetrahydrofuran; n-heptane; toluene; Heating;
|
|
|
triethylamine; Trimethylacetic acid; In tetrahydrofuran; hexane; at 50 ℃; Product distribution / selectivity; Heating / reflux;
|
|
|
hydrogenchloride; triethylamine; In tetrahydrofuran; tert-butyl methyl ether; at 50 ℃; Product distribution / selectivity; Heating / reflux;
|
|
|
triethylamine; Trimethylacetic acid; In tetrahydrofuran; tert-butyl methyl ether; at 50 ℃; Product distribution / selectivity; Heating / reflux;
|
|
|
N-ethyl-N,N-diisopropylamine; Trimethylacetic acid; In toluene; at 50 ℃; Product distribution / selectivity; Heating / reflux;
|
|
|
sodium hydroxide; Trimethylacetic acid; In toluene; at 50 ℃; Product distribution / selectivity; Heating / reflux;
|
|
|
sodium hydroxide; Trimethylacetic acid; In tetrahydrofuran; tert-butyl methyl ether; at 50 ℃; Product distribution / selectivity; Heating / reflux;
|
|
|
zinc diacetate; In tetrahydrofuran; tert-butyl methyl ether; at 50 ℃; Product distribution / selectivity; Heating / reflux;
|
|
|
N-ethylmorpholine;; Trimethylacetic acid; In toluene; at 50 ℃; Product distribution / selectivity; Heating / reflux;
|
|
|
calcium hydroxide; Trimethylacetic acid; In tetrahydrofuran; tert-butyl methyl ether; at 50 ℃; Product distribution / selectivity; Heating / reflux;
|
|
|
tert-butyl [(4R,6R)-6-aminoethyl-2,2-dimethyl-1,3-dioxan-4-yl]acetate; 2-[2-(4-fluorophenyl)-2-oxo-1-phenylethyl]-4-methyl-3-oxopentanoic acid phenylamide; In cyclohexane; at 25 - 30 ℃; for 0.25 - 0.333333h;
With Trimethylacetic acid; In cyclohexane; Heating / reflux;
|
|
|
With Trimethylacetic acid; In tetrahydrofuran; hexane; at 75 ℃; for 96h; Product distribution / selectivity;
|
|
|
With Trimethylacetic acid; In cyclohexane; at 20 ℃; Heating / reflux;
|
|
|
tert-butyl [(4R,6R)-6-aminoethyl-2,2-dimethyl-1,3-dioxan-4-yl]acetate; 2-[2-(4-fluorophenyl)-2-oxo-1-phenylethyl]-4-methyl-3-oxopentanoic acid phenylamide; Trimethylacetic acid; In tetrahydrofuran; cyclohexane; at 25 - 85 ℃; for 18h; Heating / reflux;
With ammonia; In tetrahydrofuran; cyclohexane; water; at 20 - 35 ℃; for 0.833333h; pH=8.5 - 9.5;
|
|
|
Trimethylacetic acid; In 2-methyl THF; for 30 - 35h; Heating / reflux;
|
|
|
With Trimethylacetic acid; In cyclohexane; isopropyl alcohol;
|
|
|
With Trimethylacetic acid; In cyclohexane; at 20 - 78 ℃;
|
|
|
tert-butyl [(4R,6R)-6-aminoethyl-2,2-dimethyl-1,3-dioxan-4-yl]acetate; 2-[2-(4-fluorophenyl)-2-oxo-1-phenylethyl]-4-methyl-3-oxopentanoic acid phenylamide; With Trimethylacetic acid; In tetrahydrofuran; n-heptane; toluene; for 40h; Heating / reflux;
With sodium hydroxide; In tetrahydrofuran; n-heptane; water; toluene;
With hydrogenchloride; In tetrahydrofuran; n-heptane; water; toluene;
|
|
|
tert-butyl [(4R,6R)-6-aminoethyl-2,2-dimethyl-1,3-dioxan-4-yl]acetate; 2-[2-(4-fluorophenyl)-2-oxo-1-phenylethyl]-4-methyl-3-oxopentanoic acid phenylamide; With Trimethylacetic acid; In tetrahydrofuran; n-heptane; toluene; for 40h; Heating / reflux;
With sodium hydroxide; In tetrahydrofuran; n-heptane; water; toluene;
With hydrogenchloride; In tetrahydrofuran; n-heptane; water; toluene;
|
|
|
With Trimethylacetic acid; In tetrahydrofuran; n-heptane; toluene; for 40h; Heating / reflux;
|
|
|
With Trimethylacetic acid; In tetrahydrofuran; n-heptane; toluene; for 40h; Heating / reflux;
|
|
|
With Trimethylacetic acid; In n-heptane; at 100 ℃;
|
|
|
|
|
|
With 2,2-dimethylpropanoic anhydride; In 2-methyltetrahydrofuran; at 150 ℃; for 4h; Temperature; Autoclave; Inert atmosphere; Green chemistry;
|
65 g |
|
With Trimethylacetic acid; In tetrahydrofuran; toluene; Reflux;
|
|
|
With Trimethylacetic acid; In tetrahydrofuran; n-heptane; for 60h; Reflux;
|
|
|
With Trimethylacetic acid; In tetrahydrofuran; hexane; toluene; Reflux;
|
125971-95-1 Upstream products
-
125971-86-0
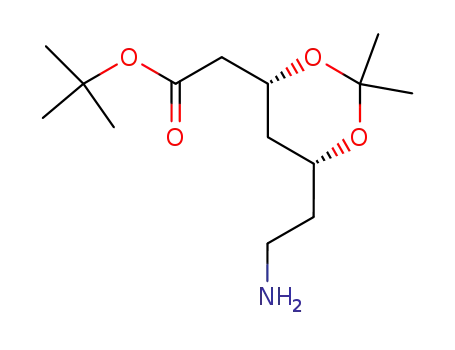
tert-butyl [(4R,6R)-6-aminoethyl-2,2-dimethyl-1,3-dioxan-4-yl]acetate
-
125971-96-2
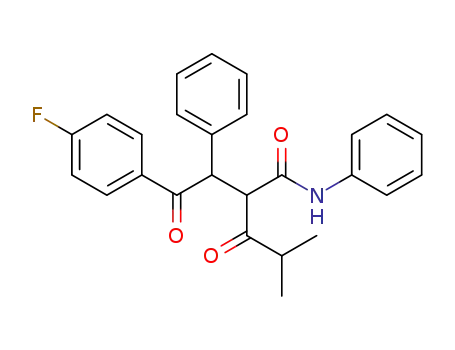
2-[2-(4-fluorophenyl)-2-oxo-1-phenylethyl]-4-methyl-3-oxopentanoic acid phenylamide
-
124401-38-3
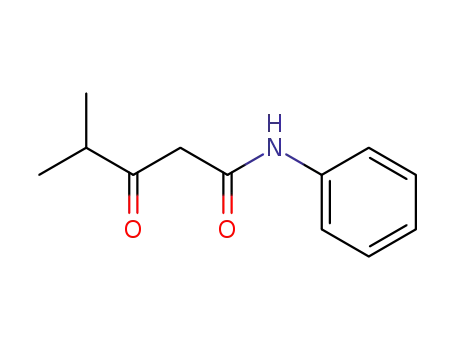
4-methyl-3-oxo-N-phenylpentanamide
-
100-52-7
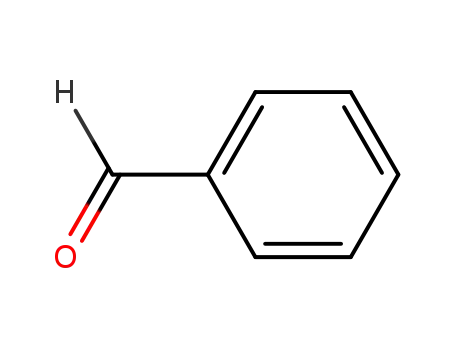
benzaldehyde
125971-95-1 Downstream products
-
134395-00-9
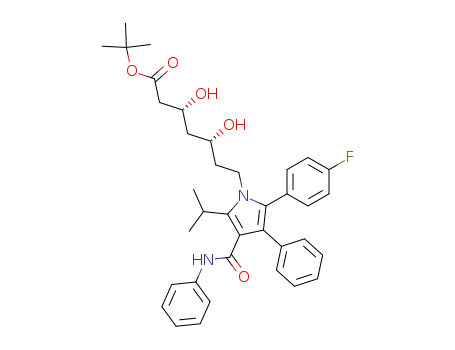
(3R,5R)-7-[2-(4-fluorophenyl)-5-isopropyl-3-phenyl-4-phenylcarbamoylpyrrol-1-yl]-3,5-dihydroxyheptanoic acid t-butyl ester
-
134523-00-5
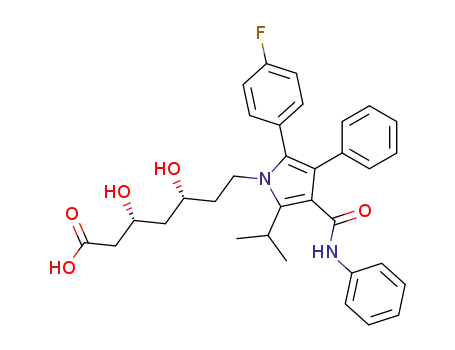
atorvastatin
-
134523-01-6
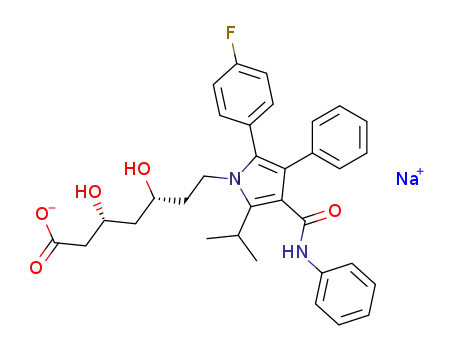
atorvastatin sodium
-
134523-03-8
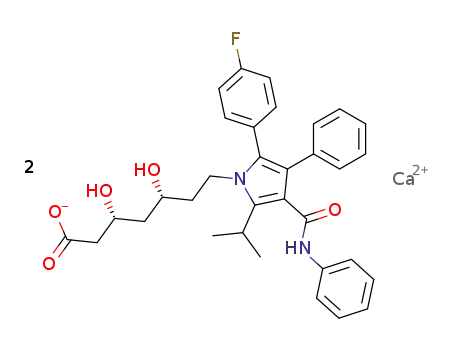
lipitor
Relevant Products
-
1,2,3-Trifluoro-4-nitrobenzene
CAS:771-69-7
-
2,4-Difluoronitrobenzene
CAS:446-35-5
-
2,4-Difluorobenzonitrile
CAS:3939-09-1