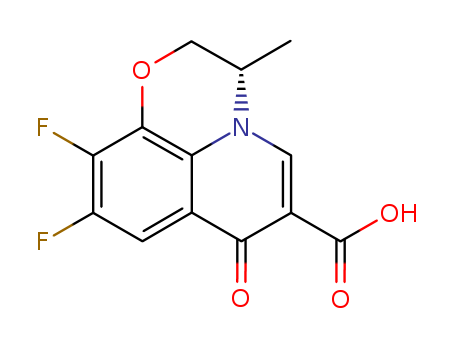
100986-89-8
- Product Name:Levofloxacin carboxylic acid
- Molecular Formula:C13H9F2NO4
- Purity:99%
- Molecular Weight:281.216
Product Details;
CasNo: 100986-89-8
Molecular Formula: C13H9F2NO4
Export Buy Reliable Quality Levofloxacin carboxylic acid 100986-89-8 Lowest Price
- Molecular Formula:C13H9F2NO4
- Molecular Weight:281.216
- Vapor Pressure:3.14E-09mmHg at 25°C
- Melting Point:>280 °C
- Refractive Index:1.635
- Boiling Point:459.2 °C at 760 mmHg
- PKA:4.87±0.40(Predicted)
- Flash Point:231.5 °C
- PSA:68.53000
- Density:1.61 g/cm3
- LogP:1.93130
Levofloxacin carboxylic acid(Cas 100986-89-8) Usage
|
Chemical Properties |
White Solid |
|
Uses |
Levofloxacin intermediate. |
InChI:InChI=1/C13H9F2NO4/c1-5-4-20-12-9(15)8(14)2-6-10(12)16(5)3-7(11(6)17)13(18)19/h2-3,5H,4H2,1H3,(H,18,19)/t5-/m0/s1
100986-89-8 Relevant articles
Novel ofloxacin derivatives: Synthesis, antimycobacterial and toxicological evaluation
Dinakaran, Murugesan,Senthilkumar, Palaniappan,Yogeeswari, Perumal,China, Arnab,Nagaraja, Valakunja,Sriram, Dharmarajan
, p. 1229 - 1236 (2008)
Thirty novel 9-fluoro-2,3-dihydro-8,10-(...
Preparation method of levofloxacin intermediate
-
Paragraph 0017; 0042-0056, (2020/09/16)
The invention relates to a preparation m...
Synthesis method of levofloxacin carboxylic acid (by machine translation)
-
Paragraph 0037-0045, (2020/02/27)
The method comprises the following steps...
ANTIBIOTIC RESISTANCE BREAKERS
-
Page/Page column 77; 78, (2019/01/05)
The invention relates to antibiotic comp...
Environment-friendly method for preparing levofloxacin hydrochloride
-
Paragraph 0019; 0023; 0027, (2017/10/05)
The invention provides an environment-fr...
100986-89-8 Process route
-
![ethyl (S)-(-)-9,10-Difluoro-3-Methyl-7-Oxo-2,3-Dihydro-7H-Pyrido[1,2,3-de]-[1,4]Benzoxazine-6-Carboxylate](/upload/2023/8/d0b8ac11-6aea-4da2-aac0-cce25b3afdcd.png)
- 106939-34-8
ethyl (S)-(-)-9,10-Difluoro-3-Methyl-7-Oxo-2,3-Dihydro-7H-Pyrido[1,2,3-de]-[1,4]Benzoxazine-6-Carboxylate

-
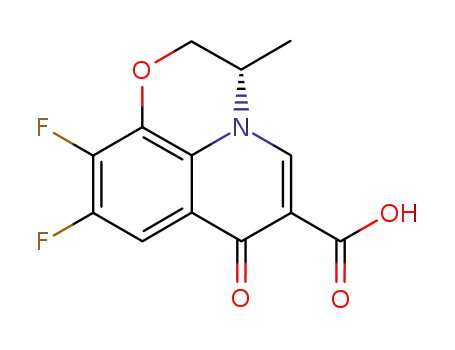
- 100986-89-8
levofloxacin Q-acid
| Conditions | Yield |
|---|---|
|
With hydrogenchloride; acetic acid;
|
91% |
|
In hydrogenchloride; acetic acid; for 0.666667h; Heating;
|
61% |
|
With hydrogenchloride; acetic acid;
|
|
|
With hydrogenchloride; water; In acetic acid; at 75 - 80 ℃; for 6h;
|
|
|
With hydrogenchloride; In acetic acid;
|
|
|
With sulfuric acid; acetic acid; In water; for 4h; Time; Reflux;
|
|
|
With sulfuric acid; acetic acid; In N,N-dimethyl-formamide; for 1h; Time; Reflux; Green chemistry;
|
4.81 g |
|
With sulfuric acid; acetic acid; In water; at 20 ℃; for 3h; Temperature; Reflux;
|
|
|
With sulfuric acid; acetic acid; In N,N-dimethyl-formamide; for 1h; Reflux;
|
|
|
With water; sodium hydroxide; In ethanol; at 20 ℃; for 1h;
|
|
|
ethyl (S)-(-)-9,10-Difluoro-3-Methyl-7-Oxo-2,3-Dihydro-7H-Pyrido[1,2,3-de]-[1,4]Benzoxazine-6-Carboxylate; With water; sodium carbonate; at 98.5 ℃; for 5h; Inert atmosphere; Large scale;
pH=4; Temperature; Reagent/catalyst; Acidic conditions; Large scale;
|
5.12 kg |
-
-
C14H11F2NO4

-

- 100986-89-8
levofloxacin Q-acid
| Conditions | Yield |
|---|---|
|
With hydrogenchloride; acetic acid; In water; for 12h;
|
95.9% |
100986-89-8 Upstream products
-
106939-34-8
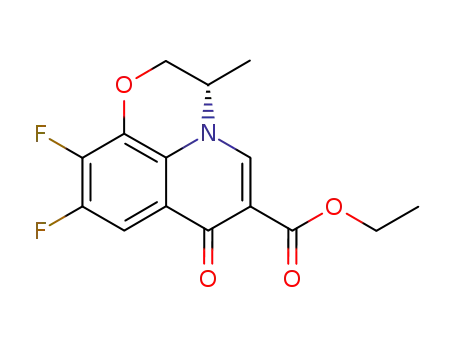
ethyl (S)-(-)-9,10-Difluoro-3-Methyl-7-Oxo-2,3-Dihydro-7H-Pyrido[1,2,3-de]-[1,4]Benzoxazine-6-Carboxylate
-
110548-03-3
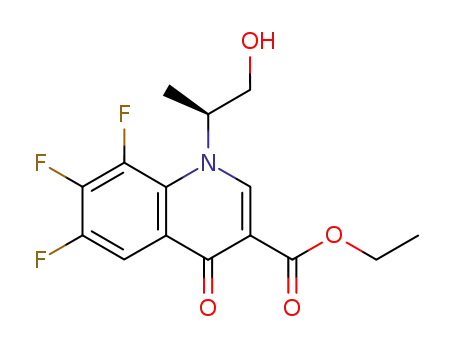
(-)-ethyl 1,4-dihydro-1-<1(S)-(hydroxymethyl)ethyl>-4-oxo-6,7,8-trifluoroquinoline-3-carboxylate
-
188058-81-3
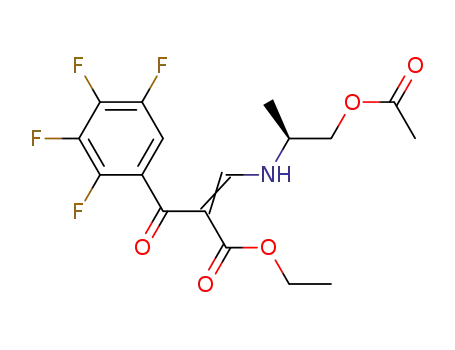
(+)-ethyl 2-(2,3,4,5-tetrafluorobenzoyl)-3-[(1-acetoxyprop-2(S)-yl)amino]acrylate
-
188058-77-7
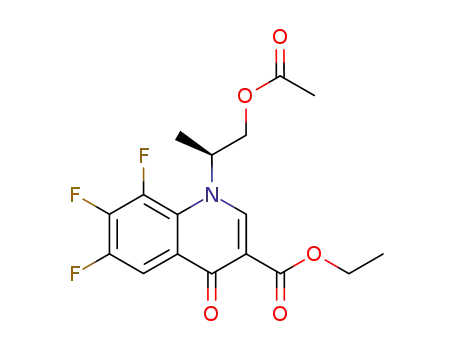
(-)-ethyl 1,4-dihydro-1-[1(S)-(acetoxymethyl)ethyl]-4-oxo-6,7,8-trifluoroquinoline-3-carboxylate
100986-89-8 Downstream products
-
100986-85-4
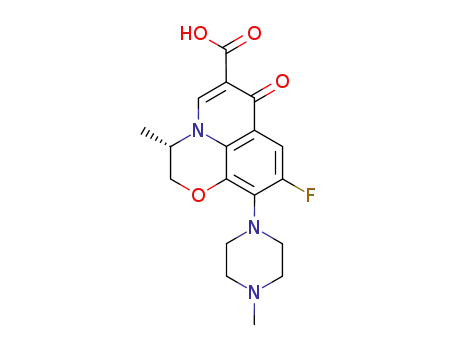
levofloxacin
-
138199-71-0
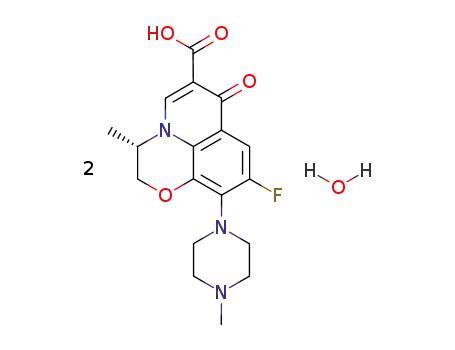
levofloxacin hemihydrate
-
117707-40-1
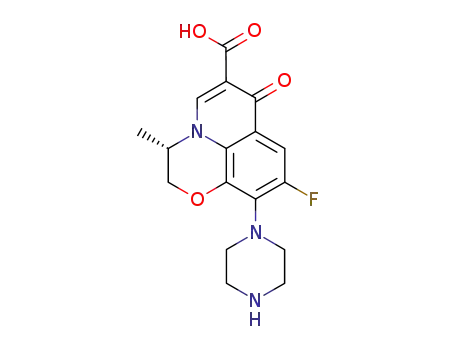
S-(-)-9-fluoro-2,3-dihydro-3-methyl-10-(1-piperazinyl)-7-oxo-7H-pyrido-<1,2,3-de><1,4>benzoxazine-6-carboxylic acid
-
1422376-41-7
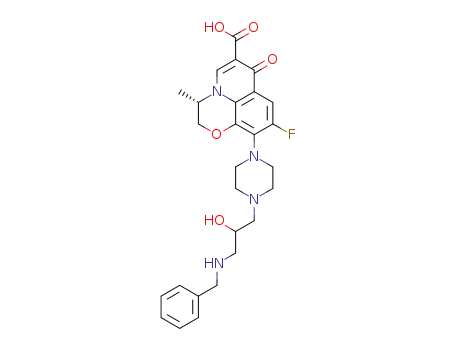
(S)-10-(4-(3-(benzylamino)-2-hydroxypropyl)piperazin-1-yl)-9-fluoro-3,7-dihydro-3-methyl-7-oxo-2H-[1,4]oxazino[2,3,4-ij]quinoline-6-carboxylic acid
Relevant Products
-
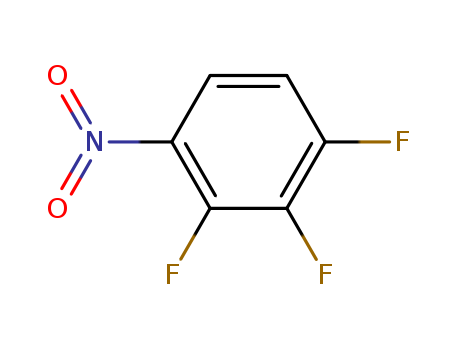
1,2,3-Trifluoro-4-nitrobenzene
CAS:771-69-7
-
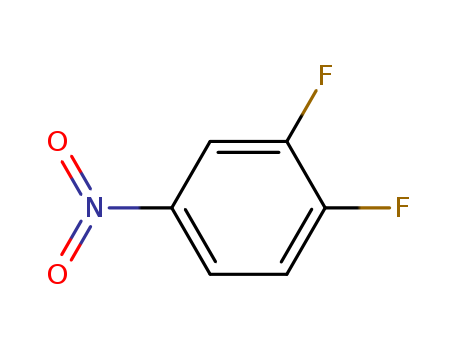
3,4-Difluoronitrobenzene
CAS:369-34-6
-
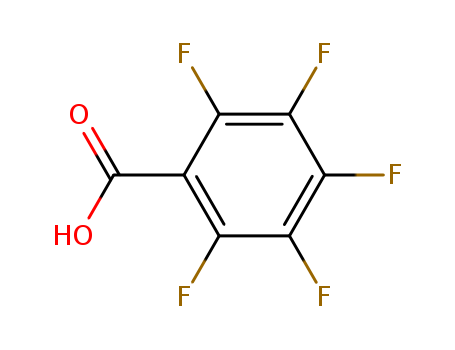
2,3,4,5,6-Pentafluorobenzoic acid
CAS:602-94-8