399-95-1
- Product Name:4-Amino-3-fluorophenol
- Molecular Formula:C6H6FNO
- Purity:99%
- Molecular Weight:127.118
Product Details;
CasNo: 399-95-1
Molecular Formula: C6H6FNO
Appearance: Brown crystal
Factory Sells Quality Manufacturer Supply 4-Amino-3-fluorophenol 399-95-1 Safe Transportation
- Molecular Formula:C6H6FNO
- Molecular Weight:127.118
- Appearance/Colour:Brown crystal
- Vapor Pressure:0.00633mmHg at 25°C
- Melting Point:135-137 °C
- Refractive Index:1.601
- Boiling Point:263.3 °C at 760 mmHg
- PKA:9.23±0.18(Predicted)
- Flash Point:113.1 °C
- PSA:46.25000
- Density:1.347 g/cm3
- LogP:1.69470
4-Amino-3-fluorophenol(Cas 399-95-1) Usage
|
Chemical Properties |
Dark Brown Solid |
InChI:InChI=1/C6H6FNO/c7-5-3-4(8)1-2-6(5)9/h1-3,9H,8H2
399-95-1 Relevant articles
A potent free fatty acid receptor 1 agonist with a glucose-dependent antihyperglycemic effect
Wang, Xuekun,Xu, Yurui,Feng, Shujun,Huang, Xinyu,Meng, Xia,Chen, Jiao,Guo, Leilei,Ge, Junliang,Zhang, Jikang,Chen, Jianmei,Cheng, Li,Gu, Kai,Zhang, Yu,Jiang, Qing,Ning, Xinghai
, p. 8975 - 8978 (2019)
We present a novel free fatty acid recep...
Benzothiazolyl ureas are low micromolar and uncompetitive inhibitors of 17Β-HSD10 with implications to Alzheimer’s disease treatment
Aitken, Laura,Benek, Ondrej,Chribek, Matej,Dolezal, Rafael,Gunn-Moore, Frank,Hrabinova, Martina,Hroch, Lukas,Jun, Daniel,Kralova, Vendula,Kuca, Kamil,Lycka, Antonin,Musilek, Kamil,Prchal, Lukas,Schmidt, Monika,Vinklarova, Lucie,Zemanova, Lucie
, (2020/03/26)
Human 17β-hydroxysteroid dehydrogenase t...
Regorafenib analogues and their ferrocenic counterparts: Synthesis and biological evaluation
Wilde, Myron,Arzur, Danielle,Baratte, Blandine,Lefebvre, Dorian,Robert, Thomas,Roisnel, Thierry,Le Jossic-Corcos, Catherine,Bach, Stéphane,Corcos, Laurent,Erb, William
supporting information, p. 19723 - 19733 (2020/12/04)
Approved by the FDA in 2012, regorafenib...
A process for preparing auspicious standard phinney intermediates
-
Paragraph 0007; 0014-0015; 0018-0019; 0024; 0029; 0034, (2019/05/06)
The invention discloses a method for pre...
399-95-1 Process route
-
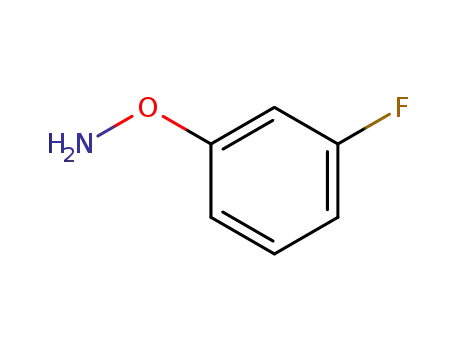
- 127682-43-3
O-(3-fluorophenyl)hydroxylamine

-
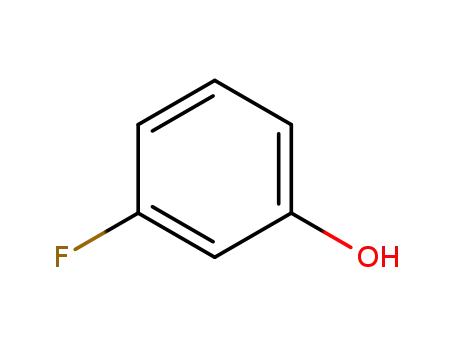
- 372-20-3
3-fluorophenol

-
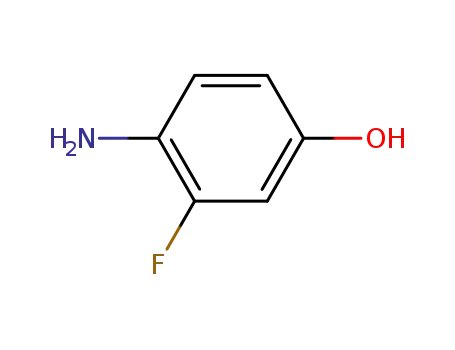
- 399-95-1
4-amino-3-fluorophenol

-
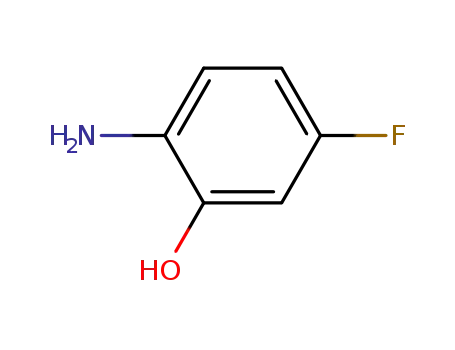
- 53981-24-1
2-amino-5-fluorophenol

-
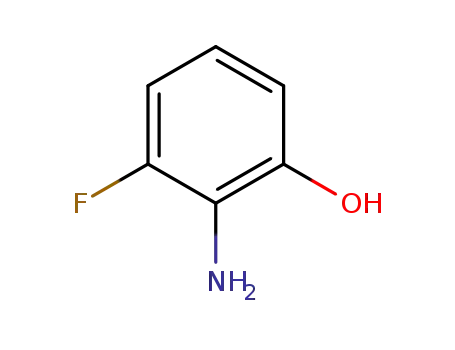
- 53981-23-0
2-amino-3-fluorophenol
| Conditions | Yield |
|---|---|
|
With trifluoroacetic acid; at 70 ℃; for 6h; Product distribution; Rate constant; Kinetics; ΔH(excit.), ΔG(excit.), ΔS(excit.);
|
-
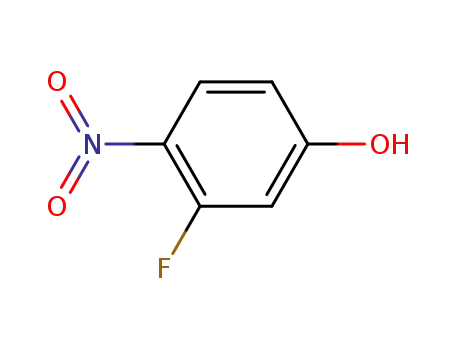
- 394-41-2
3-fluoro-4-nitrophenol

-

- 399-95-1
4-amino-3-fluorophenol
| Conditions | Yield |
|---|---|
|
With hydrogen; palladium 10% on activated carbon; In tetrahydrofuran; ethanol; at 20 ℃; for 4.5h;
|
100% |
|
With iron; acetic acid; In ethyl acetate; for 3h; Heating / reflux;
|
98% |
|
With hydrogen; palladium 10% on activated carbon; In ethyl acetate; for 4h;
|
97% |
|
With hydrogen; palladium 10% on activated carbon; In ethyl acetate; for 4h;
|
97% |
|
With hydrogen; palladium 10% on activated carbon; In ethyl acetate; for 4h;
|
97% |
|
With hydrogen; palladium 10% on activated carbon; In ethyl acetate; for 4h;
|
97% |
|
With hydrogen; palladium 10% on activated carbon; In ethyl acetate; for 4h;
|
97% |
|
With palladium 10% on activated carbon; hydrogen; In ethanol; ethyl acetate; at 20 ℃; for 5h;
|
96% |
|
With hydrogen; palladium 10% on activated carbon; In ethyl acetate;
|
96% |
|
With palladium 10% on activated carbon; hydrogen; In tetrahydrofuran; at 20 ℃; for 8h; under 2250.23 Torr; Pressure; Temperature; Solvent; Autoclave;
|
95% |
|
With palladium on activated charcoal; hydrogen; In ethyl acetate; for 16h;
|
94% |
|
|
92% |
|
With hydrogen; palladium 10% on activated carbon; In ethanol; at 20 ℃; for 3h; under 2068.65 Torr;
|
90% |
|
With hydrogen; palladium 10% on activated carbon; In ethanol; at 20 ℃; for 16h;
|
82% |
|
With palladium 10% on activated carbon; potassium formate; In tetrahydrofuran; water; at 50 ℃; for 5h;
|
72.3% |
|
With palladium 10% on activated carbon; potassium formate; In tetrahydrofuran; water; at 50 ℃; for 5h;
|
72.3% |
|
With iron; ammonium chloride; In ethanol; water; at 70 ℃; for 12h;
|
61% |
|
With hydrogenchloride; ethanol; iron;
|
|
|
With hydrogen; palladium on activated charcoal;
|
|
|
With hydrogen; palladium 10% on activated carbon; In ethanol; for 2h; under 1125.11 Torr;
|
|
|
With ammonium formate; palladium 10% on activated carbon; In tetrahydrofuran; methanol; at 20 ℃; for 1h;
|
|
|
With sodium sulfide; for 0.0833333h; Time; Microwave irradiation; Ionic liquid;
|
|
|
With palladium 10% on activated carbon; hydrogen; In tetrahydrofuran; ethanol; for 16h;
|
|
|
With tetrabutoxytitanium; hydrogen; nickel(II) nitrate; palladium dichloride; In ethanol; at 45 ℃; for 2h; under 1500.15 Torr;
|
|
|
With sodium tetrahydroborate; water; triethylamine; Reagent/catalyst;
|
|
|
With palladium on activated charcoal; hydrogen; In ethanol; at 20 ℃;
|
|
|
With palladium 10% on activated carbon; hydrogen; In ethanol; at 20 ℃;
|
1.13 g |
399-95-1 Upstream products
-
394-41-2

3-fluoro-4-nitrophenol
-
326-18-1
3-fluoro-4-phenylazo-phenol
-
1493-27-2
ortho-nitrofluorobenzene
-
127682-43-3

O-(3-fluorophenyl)hydroxylamine
399-95-1 Downstream products
-
156079-13-9
N,N-bis-(2-hydroxyethyl)-2-fluoro-4-hydroxyaniline
-
911297-02-4
N-(2-fluoro-4-hydroxyphenyl)carbamic acid tert-butyl ester
-
1020172-98-8
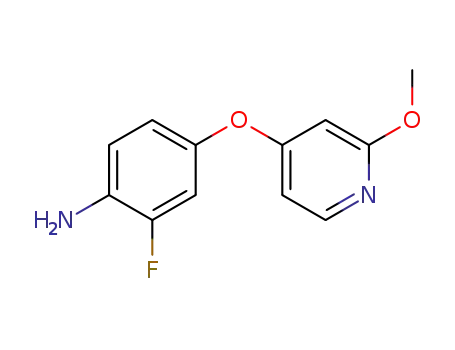
2-fluoro-4-(2-methoxypyridin-4-yloxy)benzenamine
-
911297-13-7
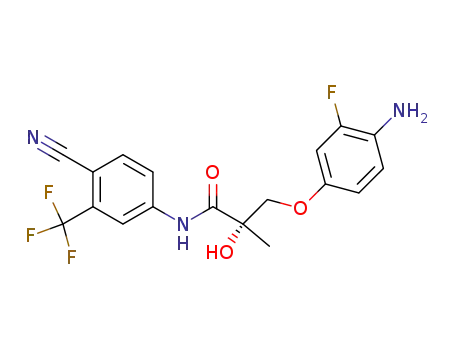
(S)-3-(4-amino-3-fluorophenoxy)-2-hydroxy-2-methyl-N-(4-cyano-3-trifluoromethylphenylcarbamoyl)propionamide
Relevant Products
-
1,2,3-Trifluoro-4-nitrobenzene
CAS:771-69-7
-
2,4,5-Trifluorophenol
CAS:2268-16-8
-
2-Chloro-6-fluorobenzaldehyde
CAS:387-45-1