387-45-1
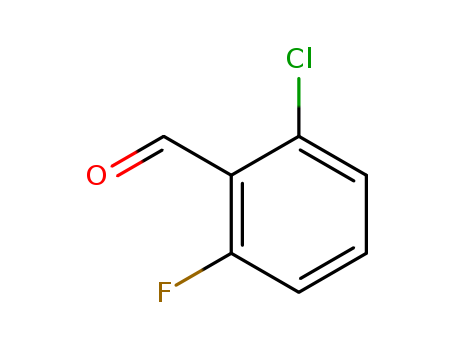
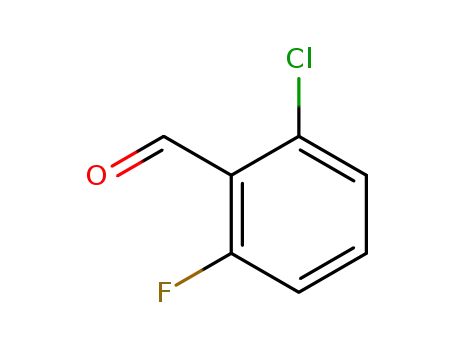
- Product Name:2-Chloro-6-fluorobenzaldehyde
- Molecular Formula:C7H4ClFO
- Purity:99%
- Molecular Weight:158.56
Product Details;
CasNo: 387-45-1
Molecular Formula: C7H4ClFO
Appearance: white to yellow solid
Top Purity Buy High Quality 2-Chloro-6-fluorobenzaldehyde 387-45-1 Efficient Shipping
- Molecular Formula:C7H4ClFO
- Molecular Weight:158.56
- Appearance/Colour:white to yellow solid
- Vapor Pressure:0.272mmHg at 25°C
- Melting Point:32-35 °C(lit.)
- Refractive Index:1.559
- Boiling Point:203.8 °C at 760 mmHg
- Flash Point:77.1 °C
- PSA:17.07000
- Density:1.352 g/cm3
- LogP:2.29160
2-Chloro-6-fluorobenzaldehyde(Cas 387-45-1) Usage
|
Chemical Properties |
whitetoyellowcrystallinesoli |
|
Uses |
2-Chloro-6-fluorobenzaldehyde was used in the synthesis of novel copolymers of methyl 2-cyano-3-dihalophenyl-2-propenoates and styrene. |
|
General Description |
2-Chloro-6-fluorobenzaldehyde undergoes piperidine catalyzed Knoevenagel condensation reaction with methyl cyanoacetate to yield methyl 2-cyano-3-dihalophenyl-2-propenoate. |
|
Consumer Uses |
ECHA has no public registered data indicating whether or in which chemical products the substance might be used. ECHA has no public registered data on the routes by which this substance is most likely to be released to the environment. |
InChI:InChI=1/C7H4ClFO/c8-6-2-1-3-7(9)5(6)4-10/h1-4H
387-45-1 Relevant articles
Latent Nucleophilic Carbenes
Marchenko, Anatoliy,Koidan, Georgyi,Hurieva, Anastasiya,Shvydenko, Kostiantyn,Rozhenko, Alexander B.,Rusanov, Eduard B.,Kyrylchuk, Andrii A.,Kostyuk, Aleksandr
, p. 373 - 385 (2021/12/27)
Using DFT and ab initio calculations, we...
Monodentate Transient Directing Group Enabled Pd-Catalyzed Ortho-C-H Methoxylation and Chlorination of Benzaldehydes
Li, Feng,Zhou, Yirong,Yang, Heng,Wang, Ziqi,Yu, Qinqin,Zhang, Fang-Lin
supporting information, p. 3692 - 3695 (2019/05/24)
We report Pd-catalyzed ortho-C-H methoxy...
Pd-Catalyzed, ortho C-H Methylation and Fluorination of Benzaldehydes Using Orthanilic Acids as Transient Directing Groups
Chen, Xiao-Yang,Sorensen, Erik J.
supporting information, p. 2789 - 2792 (2018/03/08)
The direct, Pd-catalyzed ortho C-H methy...
Stable TEMPO and ABNO Catalyst Solutions for User-Friendly (bpy)Cu/Nitroxyl-Catalyzed Aerobic Alcohol Oxidation
Steves, Janelle E.,Stahl, Shannon S.
, p. 11184 - 11188 (2015/11/18)
Two solutions, one consisting of bpy/TEM...
387-45-1 Process route
-

- 56456-50-9
(2-chloro-6-fluorophenyl)methanol

-
- 387-45-1
2-chloro-6-fluorobenzaldehyde
| Conditions | Yield |
|---|---|
|
With 4-methyl-morpholine; chromium(VI) oxide; hydrogenchloride; In diethyl ether; chloroform; at 65 ℃; for 0.1h; microwave irradiation;
|
98% |
|
With 1-methyl-1H-imidazole; [2,2]bipyridinyl; 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; tetrakis(acetonitrile)copper(I)tetrafluoroborate; In acetonitrile; at 20 ℃;
|
98% |
|
With dmap; 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; copper diacetate; In neat (no solvent); at 80 ℃; for 18h; Green chemistry;
|
95% |
|
With 1-methyl-1H-imidazole; [2,2]bipyridinyl; 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; tetrakis(acetonitrile)copper(I) trifluoromethanesulfonate; In acetonitrile; at 20 - 50 ℃; for 2.5h; chemoselective reaction; Sealed tube;
|
94% |
|
With 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; sodium carbonate; N-Phenylglycine; copper(ll) bromide; In water; for 8h; Reflux; Schlenk technique;
|
91% |
|
With dipotassium peroxodisulfate; at 55 ℃; for 0.25h; Ionic liquid;
|
87% |
|
With tert.-butylhydroperoxide; tetrabutylammomium bromide; In decane; benzene; at 40 ℃; for 24h; chemoselective reaction; Inert atmosphere; Sealed tube;
|
75% |
-
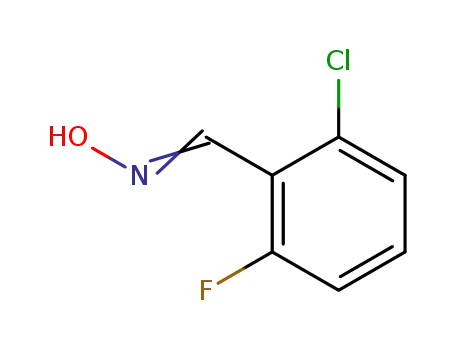
![N-[(2-chloro-6-fluorophenyl)methylidene]hydroxylamine](/upload/2023/8/da356b9d-e894-49bd-b407-7722eadea6be.png)
- 443-33-4
N-[(2-chloro-6-fluorophenyl)methylidene]hydroxylamine

-

- 387-45-1
2-chloro-6-fluorobenzaldehyde
| Conditions | Yield |
|---|---|
|
With iron(III) chloride; 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; oxygen; In water; toluene; at 60 ℃; for 5h; under 760.051 Torr;
|
87% |
387-45-1 Upstream products
-
443-83-4

2-chloro-6-fluorotoluene
-
83-38-5

2,6-dichlorobenzaldehyde
-
7726-95-6

bromine
-
75-15-0

carbon disulfide
387-45-1 Downstream products
-
392-22-3

6-fluoro-2-chloro-trans-cinnamic acid
-
625-98-9

3-chlorofluorobenzene
-
443-33-4
N-[(2-chloro-6-fluorophenyl)methylidene]hydroxylamine
-
343-76-0
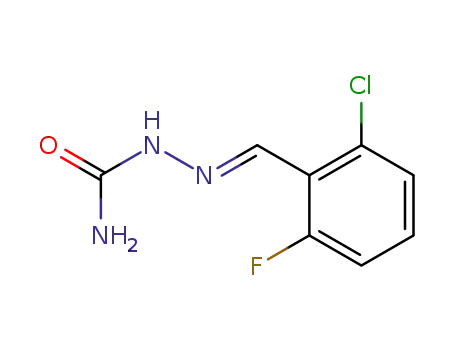
2-chloro-6-fluoro-benzaldehyde-semicarbazone
Relevant Products
-
Metamifop
CAS:256412-89-2
-
4-Amino-3-fluorophenol
CAS:399-95-1
-
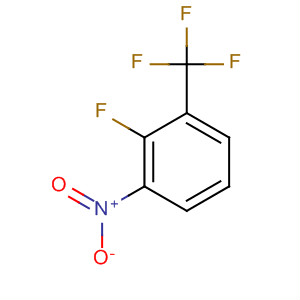
2-fluoro-3-nitrobenzetrifluoride
CAS:61324-97-8