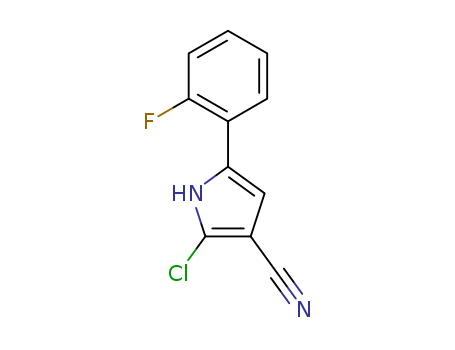
1240948-72-4
- Product Name:2-Chloro-5-(2-fluorophenyl)-1h-pyrrole-3-carbonitrile
- Molecular Formula:
- Purity:99%
- Molecular Weight:220.633
Product Details;
CasNo: 1240948-72-4
Buy Best Quality 2-Chloro-5-(2-fluorophenyl)-1h-pyrrole-3-carbonitrile 1240948-72-4 with Reasonable Price
- Molecular Formula:C11H6ClFN2
- Molecular Weight:220.633
- Boiling Point:403.0±45.0 °C(Predicted)
- PKA:12.88±0.50(Predicted)
- Density:1.41±0.1 g/cm3(Predicted)
1240948-72-4 Relevant articles
Identification, characterization, synthesis of major metabolites biotransformed from vonoprazan fumarate
Hu, Ji'an,Kou, Jingping,Li, Jianbing,Li, Yanhua,Lin, Biyue,Wang, Zhongqing,Wu, Shuming,Xiao, Qingbo,Xin, Libo,Zhou, Xinglin,Zhu, Zhu
, (2022/02/10)
A first synthetic research concerning fo...
Synthesis method of 5-(2-fluorophenyl)-1H-pyrrole-3-formaldehyde
-
Paragraph 0023; 0025; 0031-0032, (2021/01/20)
The invention discloses a synthetic meth...
Substituted pyrrole-4-alkylamine compounds and application thereof
-
Paragraph 0151-0154, (2018/10/19)
The invention relates to substituted pyr...
Preparation method of pyrrole compound
-
Paragraph 0042; 0047; 0051; 0055; 0057-0059; 0061, (2018/03/25)
The invention provides a preparation met...
1240948-72-4 Process route
-
![2-[2-(2-fluorophenyl)-2-oxoethyl]propanedinitrile](/upload/2023/8/3fdb5a19-ab1d-49a2-92fa-0b9cc85a8082.png)
- 312307-38-3
2-[2-(2-fluorophenyl)-2-oxoethyl]propanedinitrile

-

- 1240948-72-4
2-chloro-5-(2-fluorophenyl)-1H-pyrrole-3-carbonitrile
| Conditions | Yield |
|---|---|
|
With hydrogenchloride; In ethyl acetate; at 20 ℃;
|
96% |
|
With hydrogenchloride; In tetrahydrofuran; water; at 60 ℃; for 4h; Temperature;
|
92.4% |
|
With oxalyl dichloride; In toluene; at 40 - 50 ℃; for 4h; Reagent/catalyst; Temperature; Solvent;
|
85% |
|
With hydrogenchloride; In tetrahydrofuran; at 55 - 65 ℃; for 3h; Product distribution / selectivity;
|
84.2% |
|
With hydrogenchloride; In ethyl acetate; at 50 - 80 ℃; for 4h;
|
78% |
|
With hydrogenchloride; In ethyl acetate; at 75 ℃; for 3h;
|
77.2% |
-

- 445-27-2
2'-Fluoroacetophenone

-

- 1240948-72-4
2-chloro-5-(2-fluorophenyl)-1H-pyrrole-3-carbonitrile
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 2 steps
1.1: bromine / ethyl acetate / 2 h / 20 °C
1.2: 3 h / 0 - 5 °C
2.1: hydrogenchloride / ethyl acetate / 3 h / 75 °C
With hydrogenchloride; bromine; In ethyl acetate;
|
|
|
Multi-step reaction with 2 steps
1.1: ethyl acetate; dihydrogen peroxide; hydrogen bromide / water / 3 h / 25 °C
1.2: 1.5 h / 0 - 5 °C
2.1: hydrogenchloride / water; tetrahydrofuran / 4 h / 60 °C
With hydrogenchloride; hydrogen bromide; dihydrogen peroxide; ethyl acetate; In tetrahydrofuran; water;
|
|
|
Multi-step reaction with 3 steps
1: copper(I) bromide / ethyl acetate / 80 °C
2: potassium hydroxide / ethanol; water / 2 h / 20 °C
3: hydrogenchloride / ethyl acetate / 20 °C
With hydrogenchloride; potassium hydroxide; copper(I) bromide; In ethanol; water; ethyl acetate;
|
|
|
Multi-step reaction with 3 steps
1: tetra-N-butylammonium tribromide / tetrahydrofuran / 20 - 30 °C
2: sodium carbonate / ethyl acetate / 5 h / 35 °C
3: hydrogenchloride / ethyl acetate / 4 h / 50 - 80 °C
With hydrogenchloride; tetra-N-butylammonium tribromide; sodium carbonate; In tetrahydrofuran; ethyl acetate;
|
1240948-72-4 Upstream products
-
312307-38-3

2-[2-(2-fluorophenyl)-2-oxoethyl]propanedinitrile
-
445-27-2

2'-Fluoroacetophenone
-
655-15-2
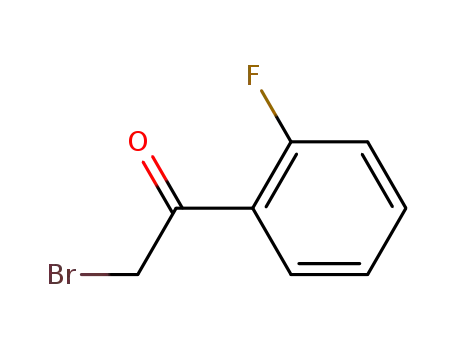
2-bromo-2'-fluoroacetophenone
1240948-72-4 Downstream products
-
2098974-13-9

1-[5-(2-fluorophenyl)-1-(pyridine-3-sulfonyl)-1H-pyrrole-3-yl]-N-methylmethylamine fumaric acid salt
-
881677-11-8

5‐(2-fluorophenyl)‐1‐(pyridine-3-sulfonyl)-1H-pyrrole-3-carbaldehyde
-
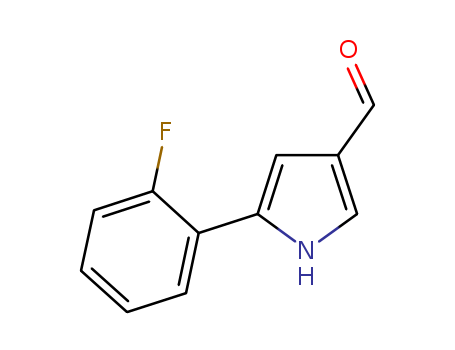
881674-56-2

5-(2-fluorophenyl)-1H-pyrrole-3-carboxaldehyde
-
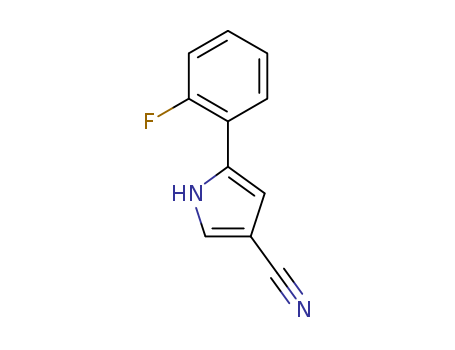
1240948-77-9

5-(2-fluorophenyl)-1H-pyrrole-3-carbonitrile
Relevant Products
-
DIISOPROPYLPHOSPHORAMIDOUS DICHLORIDE
CAS:921-26-6
-
Choline dihydrogen phosphate
CAS:83846-92-8
-
5-(2-fluorophenyl)-1H-Pyrrole-3-carboxaldehyde
CAS:881674-56-2
-
5-(2-Fluorophenyl)-1H-Pyrrole-3-Carbonitrile
CAS:1240948-77-9