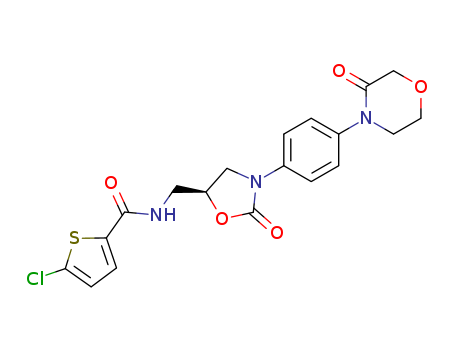
366789-02-8
- Product Name:Rivaroxaban
- Molecular Formula:C19H18ClN3O5S
- Purity:99%
- Molecular Weight:435.888
Product Details;
CasNo: 366789-02-8
Molecular Formula: C19H18ClN3O5S
Buy High Quality Rivaroxaban 366789-02-8, Hot Sale with Cheapest Price
- Molecular Formula:C19H18ClN3O5S
- Molecular Weight:435.888
- Vapor Pressure:0mmHg at 25°C
- Melting Point:228-229°C
- Refractive Index:1.633
- Boiling Point:732.609 °C at 760 mmHg
- PKA:13.36±0.46(Predicted)
- Flash Point:396.868 °C
- PSA:116.42000
- Density:1.46 g/cm3
- LogP:3.04080
Rivaroxaban(Cas 366789-02-8) Usage
|
Description |
Rivaroxaban is a pure (S)-enantiomer. It is an odorless, non-hygroscopic, white to yellowish powder. |
|
Indications and Usage |
Rivaroxaban is an antithrombotic drug and was developed in a collaboration between the German Bayer Pharmaceuticals and American Johnson company. It is different from the traditional antithrombotic drug heparin in that Rivaroxaban does not need the participation of antithrombin III and can directly antagonize free and bound Xa factors. It is also used to prevent patients with nonvalvular atrial fibrillation from cerebral apoplexy and noncentral nervous system embolism, and it can lower the recurrence risk of coronary syndromes. |
|
Uses |
Rivaroxaban is recommended as an option for treating pulmonary embolism and preventing recurrent deep vein thrombosis and pulmonary embolism in adults. Rivaroxaban (Xarelto, Bayer) is indicated for the 'treatment of deep vein thrombosis and pulmonary embolism, and prevention of recurrent deep vein thrombosis and pulmonary embolism in adults'. For the initial treatment of acute pulmonary embolism, the recommended dosage of rivaroxaban is 15 mg twice daily for the first 21 days followed by 20 mg once daily for continued treatment and prevention of recurrent venous thromboembolism. |
|
Adverse Reactions |
Bleeding events (may be serious or fatal), back pain, wound secretion, pruritus, pain in extremity, abdominal pain, blister. |
|
Chemical Properties |
White Solid |
|
Originator |
Bayer (Germany) |
|
Definition |
ChEBI: Rivaroxaban is a monocarboxylic acid amide obtained by formal condensation of the carboxy group of 5-chlorothiophene-2-carboxylic acid with the amino group of 4-{4-[(5S)-5-(aminomethyl)-2-oxo-1,3-oxazolidin-3-yl]phenyl}morpholin-3-one. An anticoagulant used for prophylaxis of venous thromboembolism in patients with knee or hip replacement surgery. It has a role as an anticoagulant and an EC 3.4.21.6 (coagulation factor Xa) inhibitor. It is a member of thiophenes, an organochlorine compound, an oxazolidinone, a member of morpholines, a lactam, an aromatic amide and a monocarboxylic acid amide. |
|
Brand name |
Xarelto |
|
Clinical Use |
Factor Xa inhibitor: Prevention of venous thromboembolism in adult patients undergoing elective hip or knee replacement surgery Treatment of DVT or PE Prophylaxis of stroke in AF Prophylaxis of atherothrombotic events in ACS |
|
Metabolism |
Metabolised by the cytochrome P450 isoenzymes CYP3A4 and CYP2J2 and by other mechanisms. About two-thirds of an oral dose is metabolised, with the metabolites excreted equally in the urine and faeces; the remaining third is excreted unchanged in the urine, mainly by active renal secretion. |
InChI:InChI=1/C19H18ClN3O5S/c20-16-6-5-15(29-16)18(25)21-9-14-10-23(19(26)28-14)13-3-1-12(2-4-13)22-7-8-27-11-17(22)24/h1-6,14H,7-11H2,(H,21,25)/t14-/m0/s1
366789-02-8 Relevant articles
New synthetic strategy for preparation of the anticoagulant drug Rivaroxaban via an asymmetric Henry reaction
Drabina, Pavel,Feixová, Viola,Sedlák, Milo?
, p. 99 - 101 (2019)
A new synthetic approach towards the ant...
Preparation method of rivaroxaban
-
, (2021/01/25)
The invention relates to a preparation m...
Preparation method of rivaroxaban
-
, (2021/03/18)
The invention relates to a preparation m...
Preparation method of rivaroxaban
-
Paragraph 0013; 0017, (2021/03/24)
The invention provides a preparation met...
Synthesis method of rivaroxaban
-
Paragraph 0012; 0034-0049, (2020/07/02)
The invention discloses a synthetic meth...
366789-02-8 Process route
-
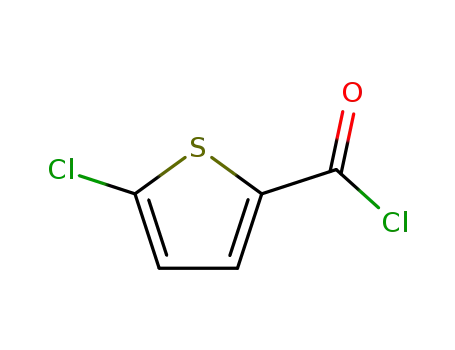
- 42518-98-9
5-chlorothiophene-2-carbonyl chloride

-
![4-{4-[(5S)-5-(aminomethyl)-2-oxo-1,3-oxazolidine-3-yl]phenyl}morpholin-3-one hydrochloride](/upload/2023/8/1e29f741-d589-4714-b85d-04e90295f678.png)
- 898543-06-1
4-{4-[(5S)-5-(aminomethyl)-2-oxo-1,3-oxazolidine-3-yl]phenyl}morpholin-3-one hydrochloride

-

- 366789-02-8,865479-71-6
Rivaroxaban
| Conditions | Yield |
|---|---|
|
With sodium carbonate; In water; acetone; toluene; at 8 - 53 ℃;
|
93.12% |
|
With sodium carbonate; In water; acetone; toluene; at 10 - 53 ℃; for 0.5h; High pressure;
|
90% |
|
With potassium carbonate; In dichloromethane; at 25 - 30 ℃; for 5h;
|
88% |
|
With potassium carbonate; In dichloromethane; at 25 - 30 ℃; for 5h;
|
88% |
|
With triethylamine; In N,N-dimethyl-formamide; at 30 - 40 ℃; for 5h;
|
86.1% |
|
With triethylamine; In N,N-dimethyl-formamide; at 30 - 40 ℃; for 5h; Concentration;
|
86.1% |
|
4-{4-[(5S)-5-(aminomethyl)-2-oxo-1,3-oxazolidine-3-yl]phenyl}morpholin-3-one hydrochloride; With triethylamine; In acetonitrile; at 20 ℃; for 0.166667h; Inert atmosphere;
5-chlorothiophene-2-carbonyl chloride; In acetonitrile; at 0 ℃; for 1.33333h; Inert atmosphere;
|
82.7% |
|
With sodium carbonate; In water; toluene; at 8 - 20 ℃; for 2h; Industrial scale;
|
80% |
|
5-chlorothiophene-2-carbonyl chloride; 4-{4-[(5S)-5-(aminomethyl)-2-oxo-1,3-oxazolidine-3-yl]phenyl}morpholin-3-one hydrochloride; With sodium carbonate; In water; acetone; toluene; at 8 - 53 ℃;
In water; acetone; toluene; at 50 - 53 ℃; for 0.5h;
|
|
|
With sodium acetate; In sulfolane; water; toluene; at 25 - 55 ℃;
|
|
|
With triethylamine; In N,N-dimethyl-formamide; at 30 - 40 ℃; for 5h;
|
150.1 g |
-
![5-chloro-N-{(R)‐2‐hydroxy‐3‐[4‐(3-oxo-4-morpholinyl)phenylamino]-propyl}thiophene-2-carboxamide](/upload/2023/8/885e43e7-b78e-43be-b3c3-f4ba1f8891c5.png)
- 721401-53-2
5-chloro-N-{(R)‐2‐hydroxy‐3‐[4‐(3-oxo-4-morpholinyl)phenylamino]-propyl}thiophene-2-carboxamide

-

- 530-62-1
1,1'-carbonyldiimidazole

-

- 366789-02-8,865479-71-6
Rivaroxaban
| Conditions | Yield |
|---|---|
|
With dmap; In acetone; at 100 ℃; for 6h;
|
95.1% |
|
In 1-methyl-pyrrolidin-2-one; toluene; at 20 - 115 ℃; for 1.33333h;
|
91.5% |
|
In 1-methyl-pyrrolidin-2-one; toluene; at 20 - 110 ℃; for 3.5h; Inert atmosphere;
|
90% |
|
In 1-methyl-pyrrolidin-2-one; toluene; at 80 - 110 ℃; for 2.5h; Inert atmosphere;
|
90% |
|
In dichloromethane; at 25 - 30 ℃; for 3h;
|
85% |
|
In ethyl acetate; at 25 - 30 ℃; for 8h;
|
85% |
366789-02-8 Upstream products
-
446292-10-0

4-[4-[(5S)-5-(aminomethyl)-2-oxo-1,3-oxazolidin-3-yl]phenyl]morpholine-3-one
-
42518-98-9

5-chlorothiophene-2-carbonyl chloride
-
446292-07-5
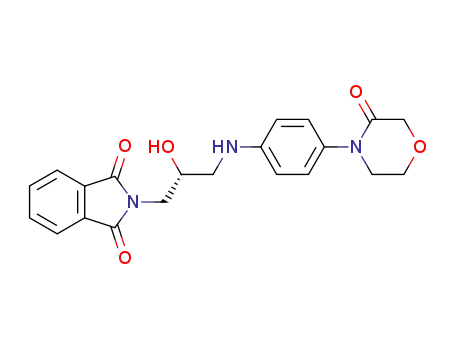
2-((2S)-2-hydroxy-3-{[4-(3-oxomorpholin-4-yl)phenyl]amino}propyl)-1H-isoindole-1,3(2H)-dione
-
446292-08-6

2-({(5S)-2-oxo-3-[4-(3-oxo-4-morpholinyl)phenyl]-1,3-oxazolidin-5-yl}methyl)-1H-isoindole-1,3(2H)-dione
366789-02-8 Downstream products
-
931117-61-2

2-({4-[(5S)-5-({[(5-chloro-2-thienyl)carbonyl]amino}methyl)-2-oxo-1,3-oxazolidin-3-yl]phenyl}-amino)ethoxyacetic acid hydrochloride
-
1009102-72-0

5-Chloro-N-(4-chlorobutanoyl)-N-({(5S)-2-oxo-3-[4-(3-oxomorpholin-4-yl)phenyl]-1,3-oxazolidin-5-yl}methyl)thiophene-2-carboxamide
-
947181-14-8
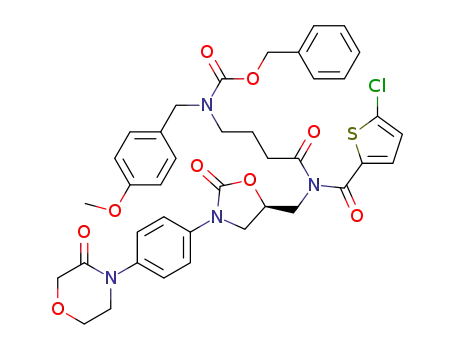
C39H39ClN4O9S
-
947181-15-9

C40H41ClN4O9S
Relevant Products
-
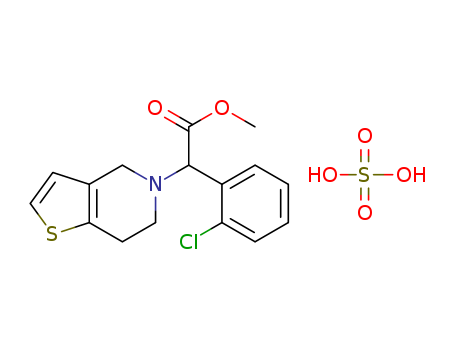
Clopidogrel hydrogen sulfate
CAS:135046-48-9
-
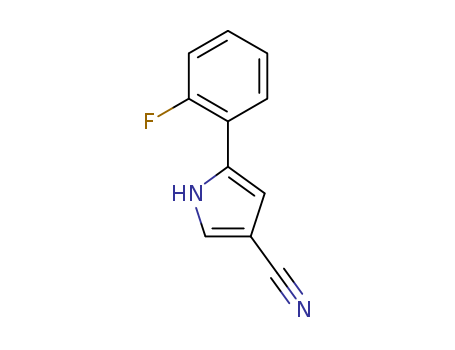
5-(2-Fluorophenyl)-1H-Pyrrole-3-Carbonitrile
CAS:1240948-77-9
-
Fluvastatin sodium salt
CAS:93957-55-2