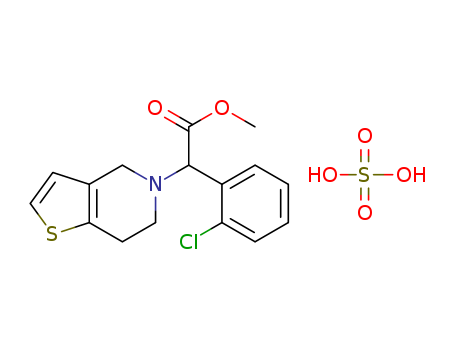
135046-48-9
- Product Name:Clopidogrel hydrogen sulfate
- Molecular Formula:C16H18ClNO6S2
- Purity:99%
- Molecular Weight:419.907
Product Details;
CasNo: 135046-48-9
Molecular Formula: C16H18ClNO6S2
Appearance: colorless to light yellow liquid
Best Quality Quality Manufacturer Supply Clopidogrel hydrogen sulfate 135046-48-9 Customized Supply
- Molecular Formula:C16H18ClNO6S2
- Molecular Weight:419.907
- Appearance/Colour:colorless to light yellow liquid
- Vapor Pressure:8.71E-09mmHg at 25°C
- Melting Point:184 °C
- Boiling Point:423.7 °C at 760 mmHg
- Flash Point:210 °C
- PSA:140.76000
- LogP:4.03980
Clopidogrel hydrogen sulfate(Cas 135046-48-9) Usage
|
Anti-platelet aggregation drug |
Thrombosis caused by arterial atheromatous plaque leads to acute thrombosis cardiovascular and cerebrovascular events. In this pathogenesis, platelets play a central role, which has been fully studied. Clopidogrel hydrogen sulfate is similar to ticlopidine, which is a new anti-platelet aggregation drug, successfully developed by French Sanofi-Synthelabo Fort for the prevention and treatment of heart, brain and other arterial circulation disorders caused by the high blood platelet aggregation, such as recent onset of stroke, myocardial infarction and patients diagnosed with peripheral arterial disease. Many countries have already used it, including United States, Europe and China. This product inhibited platelet activation by inhibiting adenosine diphosphate (ADP) pathway to inhibit platelet aggregation. Since clopidogrel hydrogen sulfate takes several days to achieve effective blood concentration, it does not apply to emergency treatment. Stop using 7 to 10 days, platelet function returned to normal state. |
|
Chemical Properties |
Colorless to light yellow liqui |
|
Uses |
Used as an antithrombotic |
|
Biochem/physiol Actions |
Inhibits ADP-induced platelet aggregation; anti-thrombotic drug. |
InChI:InChI=1/C15H14ClNO2S.H2O4S/c16-12-4-2-1-3-11(12)14(15(18)19)17-7-5-13-10(9-17)6-8-20-13;1-5(2,3)4/h1-4,6,8,14H,5,7,9H2,(H,18,19);(H2,1,2,3,4)/t14-;/m0./s1
135046-48-9 Relevant articles
Efficient Synthesis of (S)-(+)-Clopidogrel Bisulfate-Capped Silver Nanoparticles
Mahmoodi, Nosrat O.,Ghavidast, Atefeh,Ashkan, Mitra,Mamaghani, Manouchehr,Zanjanchi, Mohammad Ali,Tabatabaeian, Khalil,Arabanian, Armin
, p. 1552 - 1557 (2016/06/09)
In this work primarily one-pot synthetic...
RACEMIZATION PROCESS FOR THE PREPARATION OF AN INTERMEDIATE OF CLOPIDOGREL HYDROGEN SULPHATE
-
Page/Page column 13, (2009/07/18)
The invention relates to a process for t...
PROCESS FOR THE PREPARATION OF POLYMORPHIC FORMS OF CLOPIDOGREL HYDROGEN SULFATE
-
Page/Page column 5, (2009/04/24)
The present invention relates to a novel...
Preparation of Clopidogrel and Its Analogues Methyl Tetrahydrothienopyridine Acetate Compuunds
-
Page/Page column 11, (2008/12/08)
The present invention disclosed a prepar...
135046-48-9 Process route
-
![6,7-dihydro-4H-thieno[3,2-c]pyridine hydrochloride](/upload/2023/8/eb74e150-fcf3-4e5b-925d-ce6c9fb83fa4.png)
- 28783-41-7
6,7-dihydro-4H-thieno[3,2-c]pyridine hydrochloride

-
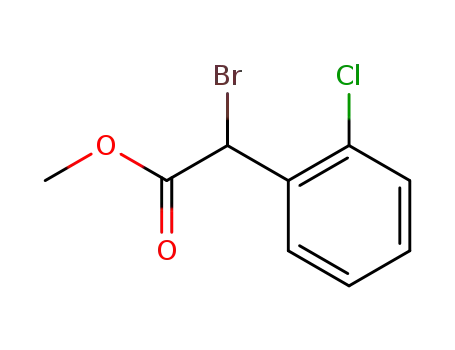
- 85259-19-4,622835-93-2,66504-71-0
methyl 2-bromo-2-(2-chlorophenyl)acetate

-
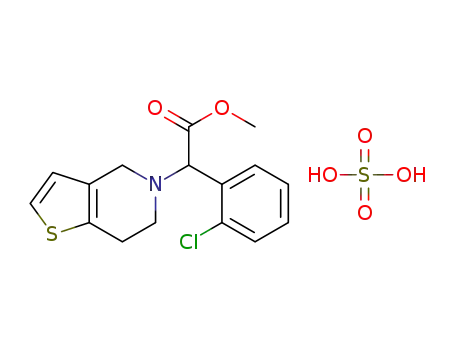
- 135046-48-9,120202-71-3,144077-07-6
clopidogrel bisulfate
| Conditions | Yield |
|---|---|
|
6,7-dihydro-4H-thieno[3,2-c]pyridine hydrochloride; With sodium carbonate; In water; at 25 ℃; for 0.5h;
methyl 2-bromo-2-(2-chlorophenyl)acetate; In water; toluene; at 20 ℃; for 12h;
With sulfuric acid; In ethyl acetate; for 1h; Reagent/catalyst; Solvent;
|
90% |
|
6,7-dihydro-4H-thieno[3,2-c]pyridine hydrochloride; methyl 2-bromo-2-(2-chlorophenyl)acetate; With potassium carbonate; In acetone;
With sulfuric acid;
|
-
![(-/+) methyl (2-chlorophenyl)-(6,7-dihydro-4H-thieno[3,2-c]pyrid-5-yl)acetate camphor sulfonate](/upload/2023/8/3ec1d12a-7f3b-4f95-a900-404718cb4805.png)
-
(-/+) methyl (2-chlorophenyl)-(6,7-dihydro-4H-thieno[3,2-c]pyrid-5-yl)acetate camphor sulfonate

-

- 135046-48-9,120202-71-3,144077-07-6
clopidogrel bisulfate
| Conditions | Yield |
|---|---|
|
(-/+) methyl (2-chlorophenyl)-(6,7-dihydro-4H-thieno[3,2-c]pyrid-5-yl)acetate camphor sulfonate; With sodium carbonate; In dichloromethane; water; at 30 - 35 ℃; pH=7.5 - 8.0;
With sulfuric acid; In acetone; at 20 - 25 ℃; for 1h;
|
80% |
135046-48-9 Upstream products
-
28783-41-7
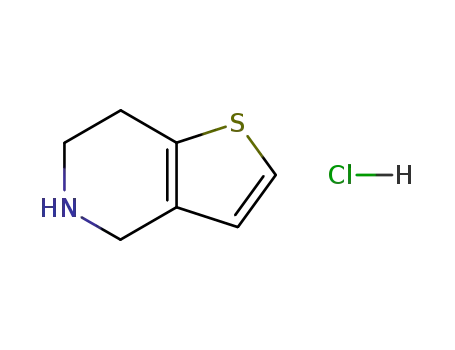
6,7-dihydro-4H-thieno[3,2-c]pyridine hydrochloride
-
85259-19-4

methyl 2-bromo-2-(2-chlorophenyl)acetate
-
90055-48-4
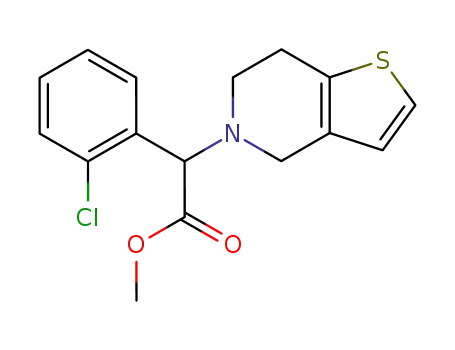
clopidogrel
-
50-00-0
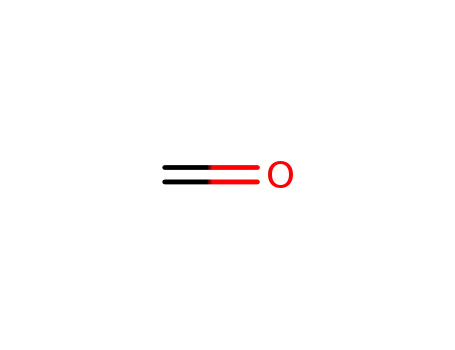
formaldehyd
135046-48-9 Downstream products
-
90055-48-4

clopidogrel
-
1376615-29-0
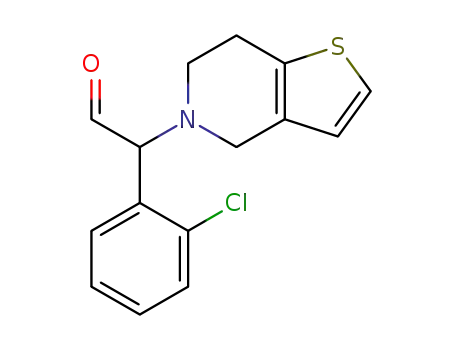
2-(2-chlorophenyl)-2-(6,7-dihydrothieno[3,2-c]pyridin-5(4H)-yl)acetaldehyde
-
1376615-30-3
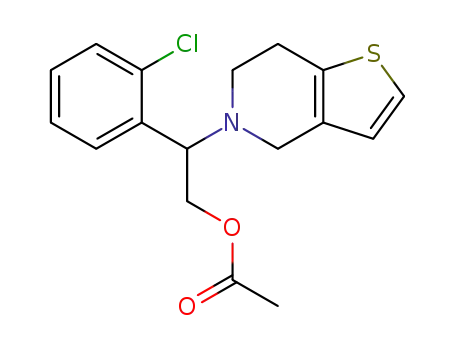
2-(2-chlorophenyl)-2-(6,7-dihydrothieno[3,2-c]pyridin-5(4H)-yl)ethyl acetate
-
1376615-31-4
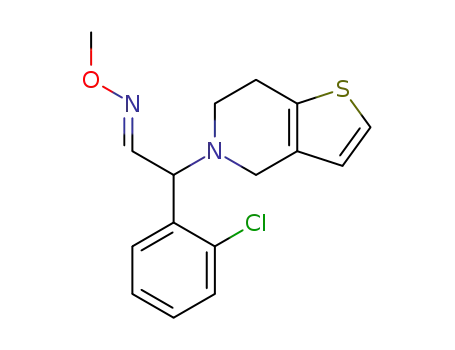
(E)-2-(2-chlorophenyl)-2-(6,7-dihydrothieno[3,2-c]pyridin-5(4H)-yl)acetaldehyde O-methyl oxime
Relevant Products
-
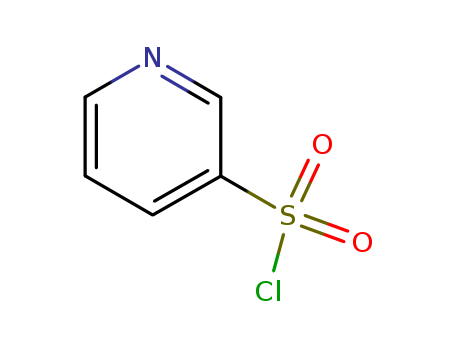
Pyridine-3-sulfonyl chloride
CAS:16133-25-8
-
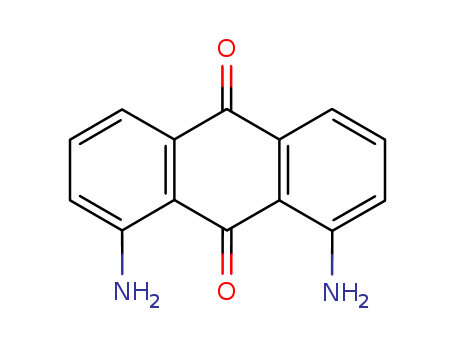
1,8-Diaminoanthraquinone
CAS:129-42-0
-
Macitentan
CAS:441798-33-0