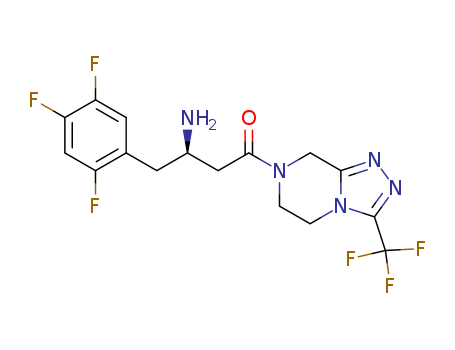
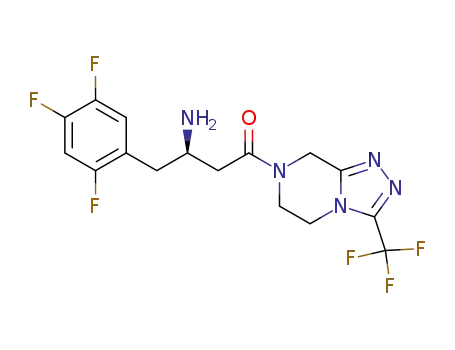
486460-32-6
- Product Name:Sitagliptin free base
- Molecular Formula:C16H15F6N5O
- Purity:99%
- Molecular Weight:407.318
Product Details;
CasNo: 486460-32-6
Molecular Formula: C16H15F6N5O
Appearance: yellow grease
Sale Factory Supply High Purity Sitagliptin free base 486460-32-6 In Bulk Supply
- Molecular Formula:C16H15F6N5O
- Molecular Weight:407.318
- Appearance/Colour:yellow grease
- Vapor Pressure:0mmHg at 25°C
- Melting Point:114.1-115.7 °C
- Refractive Index:1.59
- Boiling Point:529.9 °C at 760 mmHg
- PKA:7.20±0.10(Predicted)
- Flash Point:274.3 °C
- PSA:77.04000
- Density:1.61 g/cm3
- LogP:2.65470
Sitagliptin free base (Cas 486460-32-6) Usage
|
Description |
Sitagliptin free base is a pharmaceutical compound with therapeutic applications in the management of type 2 diabetes mellitus (T2DM). |
|
Mechanism of action |
Sitagliptin free base is an orally-bioavailable selective Dipeptidyl Peptidase-4 (DPP-4) inhibitor. Sitagliptin works by inhibiting the enzyme DPP-4, which plays a role in metabolizing incretin hormones, particularly glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP). |
|
Originator |
Merck (US) |
|
Uses |
Sitagliptin was approved by the U.S. Food and Drug Administration (FDA) in 2006. It can be used as monotherapy or in combination with other oral diabetes medications when diet and exercise are not sufficient to control blood glucose levels. Clinical trials have shown that Sitagliptin improves glycemic control by reducing both fasting and postprandial (after-meal) glucose concentrations. |
|
Brand name |
Januvia |
|
Metabolism |
Sitagliptin undergoes minimal metabolism, mainly by the cytochrome P450 isoenzyme CYP3A4, and to a lesser extent by CYP2C8. Renal excretion of sitagliptin involves active tubular secretion; it is a substrate for organic anion transporter-3 and P-glycoprotein. |
InChI:InChI=1/C16H15F6N5O/c17-10-6-12(19)11(18)4-8(10)3-9(23)5-14(28)26-1-2-27-13(7-26)24-25-15(27)16(20,21)22/h4,6,9H,1-3,5,7,23H2/t9-/m0/s1
486460-32-6 Relevant articles
Synthesis of sitagliptin, the active ingredient in Januvia® and Janumet®
Dr. Peter J. Dunn, Dr. Andrew S. Wells, Dr. Michael T. Williams
, Green Chemistry in the Pharmaceutical Industry, 10 March 2010
The hydrogenolysis of PGA-amine 17 was initially accomplished using Pearlman’s catalyst to afford sitagliptin free base 1 in 92% assay yield.
Identification of ammonium chloride as an effective promoter of the asymmetric hydrogenation of a β-enamine amide
Clausen, Andrew M.,Dziadul, Brianne,Cappuccio, Kristine L.,Kaba, Mahmoud,Starbuck, Cindy,Hsiao, Yi,Dowling, Thomas M.
, p. 723 - 726 (2006)
An investigation into the cause of subst...
Synthesis of Sitagliptin Intermediate by a Multi-Enzymatic Cascade System Using Lipase and Transaminase With Benzylamine as an Amino Donor
Taresh P. Khobragade Sharad Sarak Amol D. Pagar Hyunwoo Jeon Pritam Giri Hyungdon Yun*
, Front. Bioeng. Biotechnol., 06 October 2021
Sitagliptin is a well-reported example of a drug containing a β-amino acid moiety as a key component. Sitagliptin, an inhibitor of dipeptidyl peptidase-4, is used as an oral antidiabetic drug to treat type 2 diabetes (Yun et al., 2004; Aschner et al., 2006; Desai, 2011; Villegas-Torres et al., 2015).
486460-32-6 Process route
-
![(R,R)-N-benzyl-N-(α-methylbenzyl)-1-(2',4',5'-trifluorophenyl)-4-oxo-4-{3''-(trifluoromethyl)-5'',6''-dihydro-1'',2'',4''-triazolo[4,3-α]pyrazin-7''(8''H)-yl}butan-2-amine](/upload/2023/8/e4b5ab29-884b-4e08-9184-597aa02756eb.png)
- 1380521-88-9
(R,R)-N-benzyl-N-(α-methylbenzyl)-1-(2',4',5'-trifluorophenyl)-4-oxo-4-{3''-(trifluoromethyl)-5'',6''-dihydro-1'',2'',4''-triazolo[4,3-α]pyrazin-7''(8''H)-yl}butan-2-amine

-
- 486460-32-6,823817-55-6
sitagliptin
| Conditions | Yield |
|---|---|
|
With 30% w/w Pd(OH)2/C; hydrogen; In methanol; for 24h; under 3800.26 Torr;
|
96% |
|
With 5%-palladium/activated carbon; hydrogen; acetic acid; In methanol; at 50 ℃; for 48h; under 19001.3 Torr;
|
95% |
-
![(3R)-3-[[(1S)-1-carboxamidophenylmethyl]amino]-1-[3-(trifluoromethyl)-6,8-dihydro-5H-[1,2,4]triazolo[4,3-a]pyrazin-7-yl]-4-(2,4,5-trifluorophenyl)butan-1-one](/upload/2023/8/77ab6905-f21d-4023-ac42-374fce8e80d3.png)
- 769195-20-2
(3R)-3-[[(1S)-1-carboxamidophenylmethyl]amino]-1-[3-(trifluoromethyl)-6,8-dihydro-5H-[1,2,4]triazolo[4,3-a]pyrazin-7-yl]-4-(2,4,5-trifluorophenyl)butan-1-one

-

- 486460-32-6,823817-55-6
sitagliptin
| Conditions | Yield |
|---|---|
|
With 20% palladium hydroxide-activated charcoal; hydrogen; acetic acid; In methanol; water; at 50 ℃; for 14h; under 7500.75 Torr;
|
85% |
486460-32-6 Upstream products
-
486460-23-5
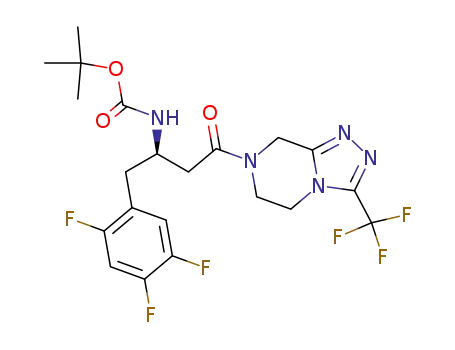
(R)-tert-butyl 4-oxo-4-(3-(trifluoromethyl)-5,6-dihydro-[1,2,4]triazolo[4,3-a]pyrazin-7(8H)-yl)-1-(2,4,5-trifluorophenyl)butan-2-ylcarbamate
-
157911-56-3
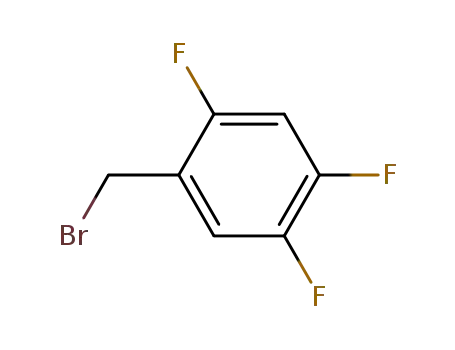
2,4,5-trifluorobenzyl bromide
-
486460-21-3
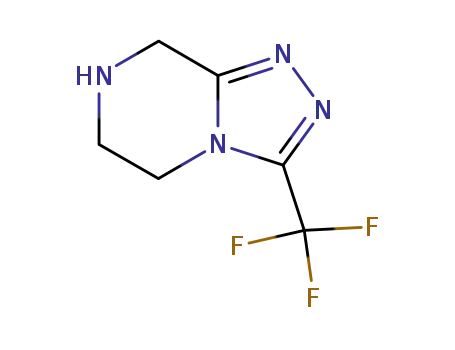
3-trifluoromethyl-5,6,7,8-tetrahydro-[1,2,4]triazolo[4,3-a]pyrazine
-
486460-00-8
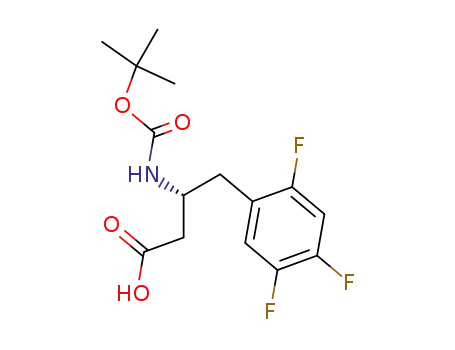
(3R)-3-[(1,1-dimethylethoxycarbonyl)amino]-4-(2,4,5-trifluorophenyl)butanoic acid
486460-32-6 Downstream products
-
654671-78-0
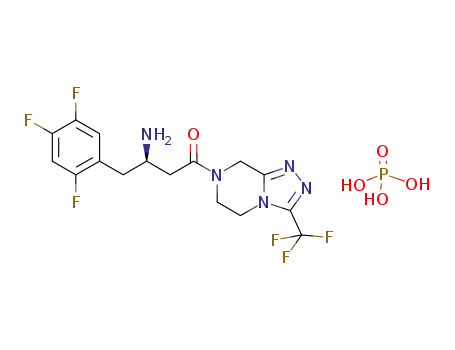
sitagliptin phosphate
-
654671-77-9
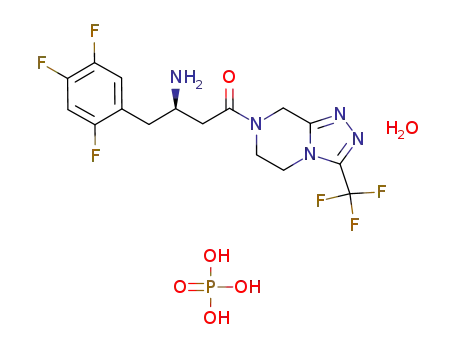
sitagliptin phosphate monohydrate
-
1169707-35-0
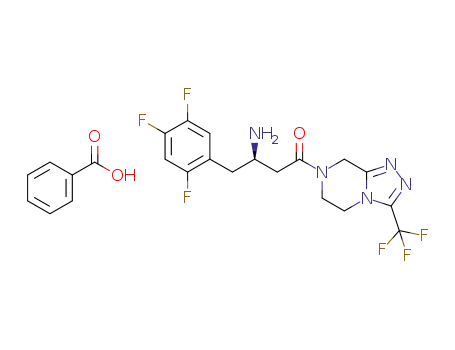
sitagliptin benzoate
-
837430-24-7
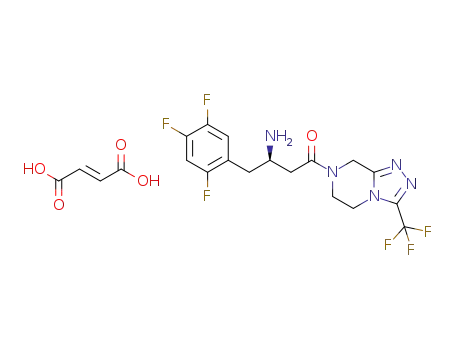
Sitagliptin fumarate
Relevant Products
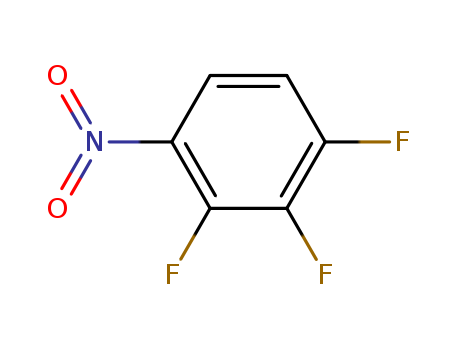
-
1,2,3-Trifluoro-4-nitrobenzene
CAS:771-69-7
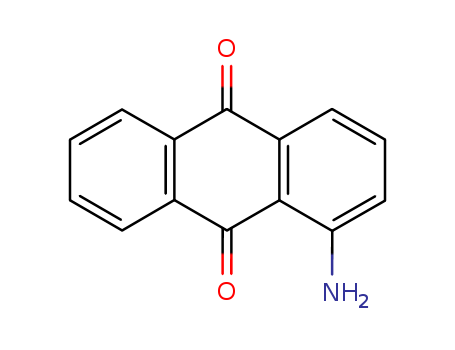
-
1-Aminoanthraquinone
CAS:82-45-1
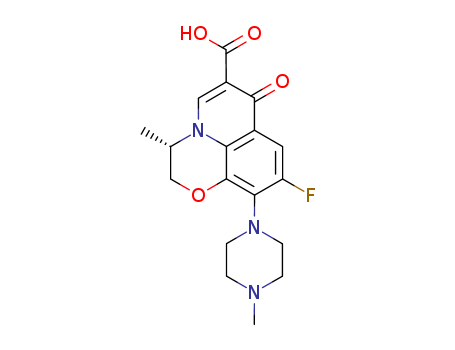
-
Levofloxacin
CAS:100986-85-4