209995-38-0
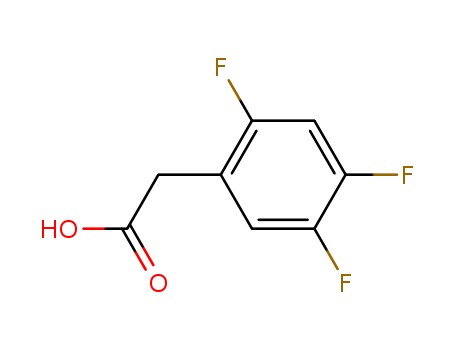
- Product Name:2,4,5-Trifluorophenylacetic acid
- Molecular Formula:C8H5F3O2
- Purity:99%
- Molecular Weight:190.122
Product Details;
CasNo: 209995-38-0
Molecular Formula: C8H5F3O2
Appearance: white or off white power
Chinese Manufacturer Supply High Purity 99% 2,4,5-Trifluorophenylacetic acid 209995-38-0 Safe Transportation
- Molecular Formula:C8H5F3O2
- Molecular Weight:190.122
- Appearance/Colour:white or off white power
- Vapor Pressure:0.00866mmHg at 25°C
- Melting Point:121-125 °C
- Refractive Index:1.488
- Boiling Point:255 °C at 760 mmHg
- PKA:3.78±0.10(Predicted)
- Flash Point:108 °C
- PSA:37.30000
- Density:1.468 g/cm3
- LogP:1.73100
2,4,5-Trifluorophenylacetic acid (Cas 209995-38-0) Usage
|
Chemical Properties |
White to light brown solid |
|
Uses |
2,4,5-Trifluorobenzeneacetic Acid is used in the synthesis of EGFR/ErbB-2-kinase inhibitors. Also used in the synthesis of new imidazopyrazinone derivatives as potnetial dipeptidyl peptidase IV inhibitors. |
|
Application |
2,4,5-Trifluorophenylacetic acid is used to synthesize the intermediate of sitagliptin, a new drug for the treatment of type II diabetes. sitagliptin is a dipeptidyl peptidase-4 (DPP-4) inhibitor newly launched by Merck. It has good curative effect, small side effects, good safety and tolerance in treating type II diabetes. |
|
Preparation |
synthesis of 2,4,5-trifluorophenylacetic acid: A 100 mL flask was charged 10.0 g of 2,4,5-trifluoromandelic acid, 23.9 g of H3PO3 (6 eq.), 0.73 g of Nal (0.1 eq.) and 0.47 g (0.10 eq.) of MSA. The obtained mixture was stirred at 95-105°C for 24 hrs. Once the conversion is completed (by HPLC; conversion > 99%, typically achieved after 24 hours), the mixture is cooled to room temperature, and 20 mL of methyl tert-butyl ether were added and then 20 mL of water where added. The obtained mixture was stirred for 5 min, then the organic layers were separated. Then 10 mL of methyl tert-butyl ether was added to the aqueous layers, stirred for 5 min, then the phase were separated. The organic layers were combined. The combined organic layers were concentrated under vacuum at 35°C, to provide crude 2,4,5-trifluorophenylacetic acid(TFPAA). To the obtained crude TFPAA was recrystallized from toluene, to obtain TFPAA, as a white crystals, 6.4 g, molar yield 69.5%, chemical purity of HPLC 99.47% A/A% |
InChI:InChI=1/C8H5F3O2/c9-5-3-7(11)6(10)1-4(5)2-8(12)13/h1,3H,2H2,(H,12,13)
209995-38-0 Relevant articles
Efficient and Straightforward Syntheses of Two United States Pharmacopeia Sitagliptin Impurities: 3-Desamino-2,3-dehydrositagliptin and 3-Desamino-3,4-dehydrositagliptin
Matej Sova, Rok Frlan, Stanislav Gobec, and Zdenko Časar*
, ACS Omega 2020, 5, 10, 5356–5364
Interestingly, synthesis of impurity 9 was reported mainly starting from sitagliptin or late stage intermediates, (4,10) while only one four-step synthetic procedure from 2,4,5-trifluorophenylacetic acid was reported in a Chinese patent application. (12) Furthermore, two additional steps are needed if 2,4,5-trifluorophenylacetic acid is prepared from readily available 1,2,4,5-tetrafluorobenzene.
Rhodium/(2S,2'S,3S,3'S)-3,3'-Di-tert-butyl-4,4'-dimethoxy-2,2',3,3'-tetrahydro-2,2'-bibenzo[d][1,3]oxaphosphole (MeO-BIBOP) Catalyzed Synthesis of (R)-3-tert-Butoxy-carbonylamino-4-(2,4,5-trifluorophenyl)butyric Acid by Asymmetric Reduction of Enamines
Li Shenga, Haoling Gaoa,b,*(), Xufeng Wua,b, Gang Fana, Pengcheng Liua
, Chinese Journal of Organic Chemistry ›› 2021, Vol. 41 ›› Issue (5): 2105-2111
It is found that [Rh(NBD)2]+ BF−4 /(2S,2'S,3S,3'S)- 3,3'-di-tert-butyl-4,4'-dimethoxy-2,2',3,3'-tetrahydro-2,2'-bibenzo[d][1,3]oxaphosphole (MeO-BIBOP) catalyst has high stereoselectivity for the hydrogenation ofN-acetylenamine ester, reaching 99% ee. 2,4,5-Trifluorophenylacetic acid (1) was used as starting material, with Mildrum?s acid (2) through condensation, alcoholysis, condensation, amino acetylation, asymmetric hydrogenation, hydrolysis and amino protection to synthesize target product with 61% yield in total and 99.62% purity.
209995-38-0 Process route
-
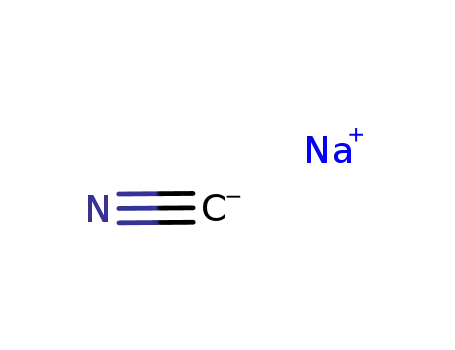
- 773837-37-9
sodium cyanide

-
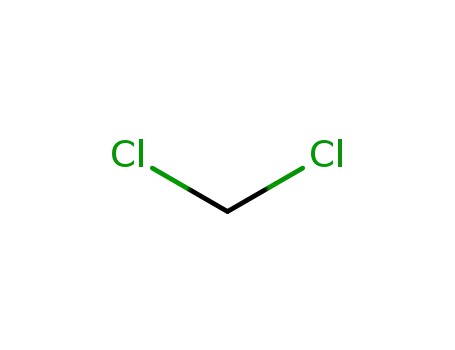
- 75-09-2
dichloromethane

-
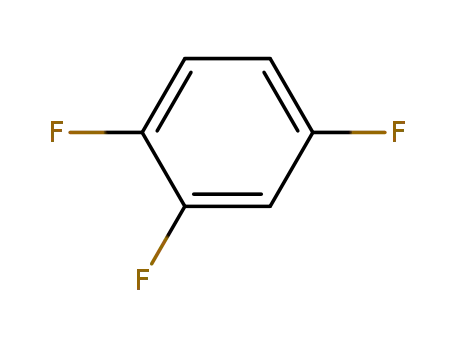
- 367-23-7
1,2,4-trifluorobenzene

-
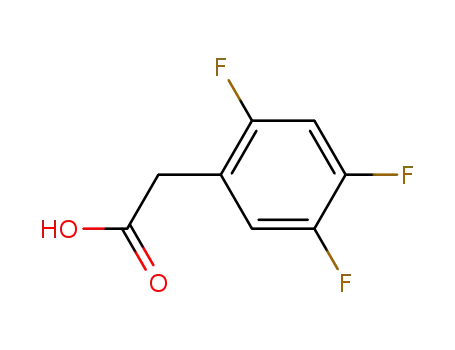
- 209995-38-0
(2,4,5-trifluorophenyl)acetic acid
| Conditions | Yield |
|---|---|
|
sodium cyanide; With sodium stearate; nitrobenzene; sodium hydroxide; In water; at 115 ℃; for 0.75h; under 3420.23 Torr; Inert atmosphere;
dichloromethane; 1,2,4-trifluorobenzene; at 6 - 142 ℃; for 6h; under 2280.15 - 5320.36 Torr; Solvent; Pressure; Reagent/catalyst; Further stages;
|
99.2% |
-
-
potassium cyanide

-

- 75-09-2
dichloromethane

-

- 367-23-7
1,2,4-trifluorobenzene

-

- 209995-38-0
(2,4,5-trifluorophenyl)acetic acid
| Conditions | Yield |
|---|---|
|
potassium cyanide; With sodium stearate; nitrobenzene; potassium hydroxide; In water; at 100 ℃; for 0.5h; under 2280.15 Torr; Inert atmosphere;
dichloromethane; 1,2,4-trifluorobenzene; at 6 - 130 ℃; under 2280.15 - 3800.26 Torr; Further stages;
|
98.2% |
209995-38-0 Upstream products
-
680993-47-9
1-allyl-2,4,5-trifluorobenzene
-
1256470-41-3
ethyl 2-(2,4,5-trifluorophenyl)acetate
-
1036273-14-9
2-(2,4,5-trifluorophenyl)-2-chloroacetic acid
-
1036273-20-7
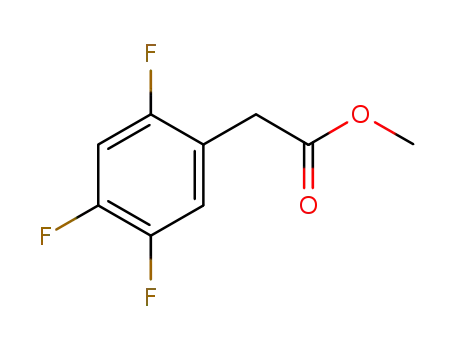
2,4,5-trifluorophenylacetic acid methyl ester
209995-38-0 Downstream products
-
764667-64-3
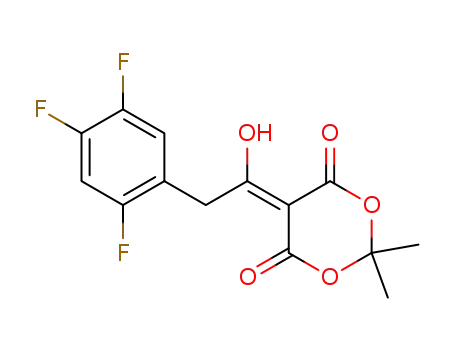
5-(1-hydroxy-2-(2,4,5-trifluorophenyl)ethylidene)-2,2-dimethyl-1,3-dioxane-4,6-dione
-
769195-26-8
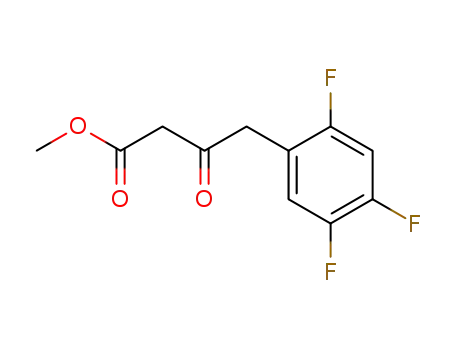
4-(2,4,5-trifluoro-phenyl)-3-oxo-butyric acid methyl ester
-
939964-30-4
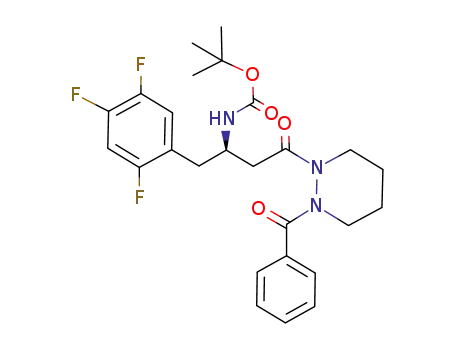
(R)-[3-(2-benzoyltetrahydropyridazin-1-yl)-3-oxo-1-(2,4,5-trifluorobenzyl)propyl]carbamic acid tert-butyl ester
-
939964-31-5
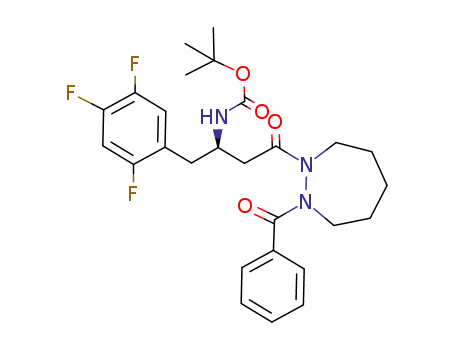
tert-butyl (R)-4-(2-benzoyl-1,2-diazepan-1-yl)-4-oxo-1-(2,4,5-trifluorophenyl)butan-2-ylcarbamate
Relevant Products
-
1,2,3-Trifluoro-4-nitrobenzene
CAS:771-69-7
-
1-Nitroanthraquinone
CAS:82-34-8
-
Norfloxacin
CAS:70458-96-7