762240-92-6
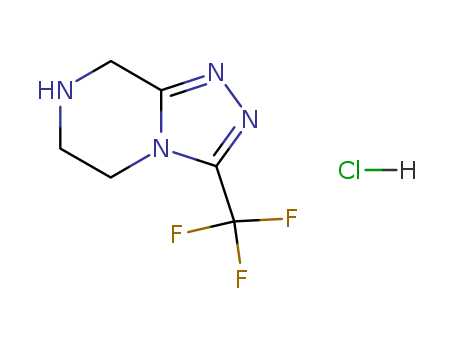
- Product Name:3-(Trifluoromethyl)-5,6,7,8-tetrahydro-[1,2,4]triazolo[4,3-a]pyrazine hydrochloride
- Molecular Formula:C6H7F3N4.HCl
- Purity:99%
- Molecular Weight:228.605
Product Details;
CasNo: 762240-92-6
Molecular Formula: C6H7F3N4.HCl
Appearance: white solid
Top Purity Factory Supply 762240-92-6 In Stock
- Molecular Formula:C6H7F3N4.HCl
- Molecular Weight:228.605
- Appearance/Colour:white solid
- Melting Point:236-246oC
- Boiling Point:266.2 °C at 760 mmHg
- Flash Point:114.8 °C
- PSA:42.74000
- LogP:1.53090
3-(Trifluoromethyl)-5,6,7,8-tetrahydro-[1,2,4]triazolo[4,3-a]pyrazine hydrochloride (Cas 762240-92-6) Usage
InChIKey: AQCSCRYRCRORET-UHFFFAOYSA-N
InChI: InChI=1S/C6H7F3N4.ClH/c7-6(8,9)5-12-11-4-3-10-1-2-13(4)5;/h10H,1-3H2;1H
762240-92-6 Relevant articles
Preparation method of sitagliptin intermediate
-
Paragraph 0024; 0030; 0034-0035; 0039-0040; 0044-0045; 0049, (2021/02/13)
The invention relates to a preparation m...
Preparation method of sitagliptin intermediate
-
Paragraph 0074-0076, (2021/06/22)
The invention discloses a preparation me...
Green synthesis method of sitagliptin intermediate
-
Paragraph 0032; 0044-0047; 0051-0052; 0056-0059, (2021/10/27)
The invention relates to a green synthes...
Preparation method of sitagliptin intermediate
-
Paragraph 0036, (2020/07/15)
The invention provides a method for prep...
762240-92-6 Process route
-
![2,2,2-trifluoro-N’-[(2Z)-piperazin-2-ylidene]acetohydrazide](/upload/2023/8/e3a98dd7-0784-4437-a299-4edb79f88cfc.png)
- 763105-70-0
2,2,2-trifluoro-N’-[(2Z)-piperazin-2-ylidene]acetohydrazide

-
![3-(trifluoromethyl)-5,6,7,8-tetrahydro-[1,2,4]triazolo[4,3-a]pyrazine hydrochloride](/upload/2023/8/74d604a2-25f0-4210-bc4e-5a5251927612.png)
- 762240-92-6
3-(trifluoromethyl)-5,6,7,8-tetrahydro-[1,2,4]triazolo[4,3-a]pyrazine hydrochloride
| Conditions | Yield |
|---|---|
|
With hydrogenchloride; In methanol; at 55 ℃; for 0.5h;
|
92% |
|
With hydrogenchloride; In ethanol; water; at 50 - 54 ℃; for 3.5h; Temperature;
|
92.6% |
|
With hydrogenchloride; In isopropyl alcohol; at 60 - 65 ℃; for 2h; Temperature; Inert atmosphere; Large scale;
|
91% |
|
With hydrogenchloride; In methanol; at 55 ℃;
|
90.7% |
|
2,2,2-trifluoro-N’-[(2Z)-piperazin-2-ylidene]acetohydrazide; In ethyl acetate; at 60 ℃; for 0.333333h; Inert atmosphere;
With hydrogenchloride; In ethyl acetate; for 3h; Inert atmosphere;
|
90.1% |
|
With hydrogenchloride; In methanol; tert-butyl methyl ether; at 20 ℃; for 1.66667 - 2.5h;
|
|
|
With hydrogenchloride; In methanol; at 55 ℃; for 0.75h;
|
|
|
With hydrogenchloride; In methanol; water; at 20 - 55 ℃; for 0.916667 - 1.75h;
|
|
|
With hydrogenchloride; In methanol; water; at 55 ℃; for 0.75h;
|
|
|
With hydrogenchloride; In methanol; at 55 ℃; for 0.75h;
|
|
|
With hydrogenchloride; In methanol; water; at 55 ℃; for 0.75h;
|
|
|
With hydrogenchloride; In methanol; water; at 55 ℃; for 0.75h;
|
|
|
2,2,2-trifluoro-N’-[(2Z)-piperazin-2-ylidene]acetohydrazide; With hydrogenchloride; In methanol; at 55 ℃; for 0.75h;
2,2,2-trifluoro-N’-[(2Z)-piperazin-2-ylidene]acetohydrazide; With hydrogenchloride; In methanol; tert-butyl methyl ether; at 20 ℃; for 1h;
|
|
|
2,2,2-trifluoro-N’-[(2Z)-piperazin-2-ylidene]acetohydrazide; With hydrogenchloride; In methanol; at 55 ℃; for 0.75h;
In tert-butyl methyl ether; at 20 ℃; for 1h;
|
|
|
With hydrogenchloride; In methanol; water; at 20 - 55 ℃; for 0.916667 - 1.75h;
|
|
|
With hydrogenchloride; In methanol; water; at 55 ℃; for 0.75h;
|
|
|
With hydrogenchloride; In methanol; water; at 20 - 55 ℃; for 0.916667 - 1.75h;
|
|
|
With hydrogenchloride; In methanol; at 50 - 55 ℃; for 3h;
|
|
|
With hydrogenchloride; In methanol; water; at 55 ℃; for 0.75h;
|
|
|
With hydrogenchloride; In methanol; water; at 55 ℃; for 0.75h;
|
|
|
With hydrogenchloride; In methanol; water; at 55 ℃; for 0.75h;
|
|
|
With hydrogenchloride; In methanol; water; at 55 ℃; for 0.75h;
|
|
|
With hydrogenchloride; In methanol; at 55 ℃; for 1h;
|
1.36 g |
-
![3-(trifluoromethyl)-[1,2,4]triazolo[4,3-a]pyrazine](/upload/2023/8/efa69587-7747-4881-9d18-55e79c0c13c3.png)
- 486460-20-2
3-(trifluoromethyl)-[1,2,4]triazolo[4,3-a]pyrazine

-
![3-(trifluoromethyl)-5,6,7,8-tetrahydro-[1,2,4]triazolo[4,3-a]pyrazine hydrochloride](/upload/2023/8/74d604a2-25f0-4210-bc4e-5a5251927612.png)
- 762240-92-6
3-(trifluoromethyl)-5,6,7,8-tetrahydro-[1,2,4]triazolo[4,3-a]pyrazine hydrochloride
| Conditions | Yield |
|---|---|
|
3-(trifluoromethyl)-[1,2,4]triazolo[4,3-a]pyrazine; With palladium on activated carbon; hydrogen; In methanol; at 40 - 45 ℃; for 24h; under 2625.26 - 3375.34 Torr;
With hydrogenchloride; In isopropyl alcohol; at 0 - 5 ℃;
|
82.9% |
|
Multi-step reaction with 2 steps
1: palladium 10% on activated carbon; hydrogen / ethanol / 45 °C / 3345.86 Torr / Large scale
2: hydrogenchloride / Isopropyl acetate / 1 h / 20 °C / Large scale
With hydrogenchloride; palladium 10% on activated carbon; hydrogen; In ethanol; Isopropyl acetate;
|
|
|
With palladium 10% on activated carbon; hydrogen; In Isopropyl acetate; at 40 - 45 ℃; under 2585.81 Torr;
|
8 g |
762240-92-6 Upstream products
-
763105-70-0
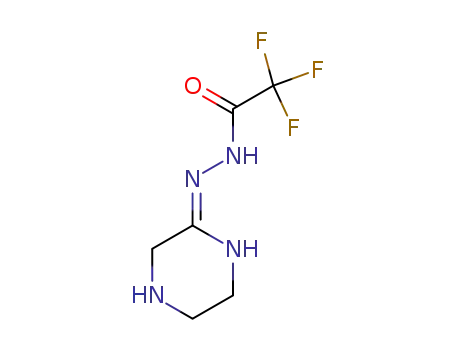
2,2,2-trifluoro-N’-[(2Z)-piperazin-2-ylidene]acetohydrazide
-
823817-55-6
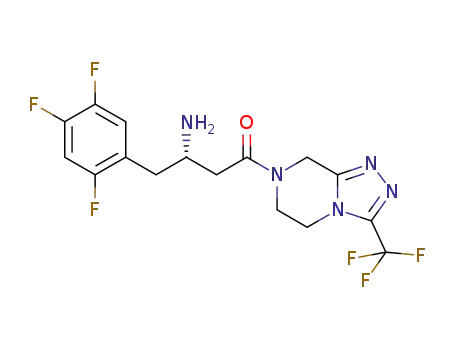
(S)-Sitagliptin
-
486460-20-2
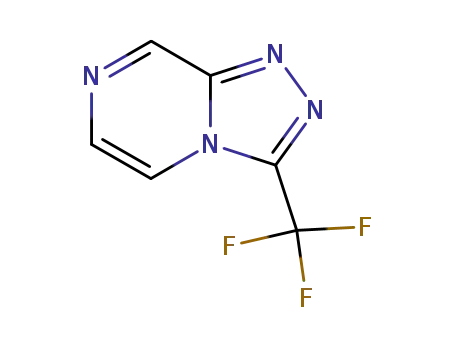
3-(trifluoromethyl)-[1,2,4]triazolo[4,3-a]pyrazine
-
486460-21-3
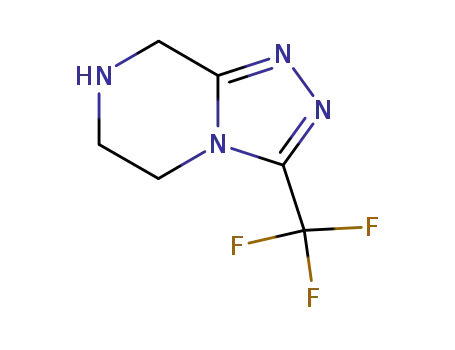
3-trifluoromethyl-5,6,7,8-tetrahydro-[1,2,4]triazolo[4,3-a]pyrazine
762240-92-6 Downstream products
-
764667-65-4
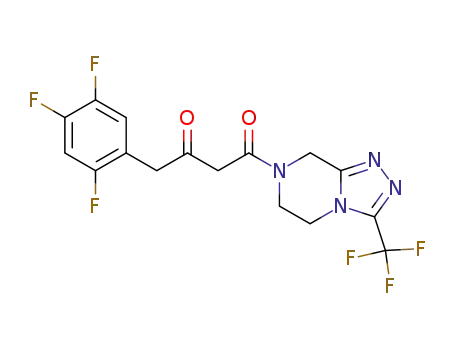
1-(3-(trifluoromethyl)-5,6-dihydro-[1,2,4]triazolo[4,3-a]pyrazin-7(8H)-yl)-4-(2,4,5-trifluorophenyl)butane-1,3-dione
-
874360-10-8
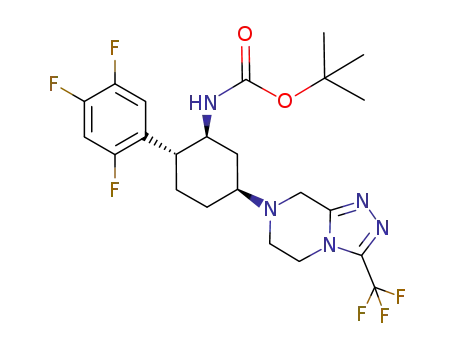
tert-butyl [(1S,2R,5S)-5-[3-(trifluoromethyl)-5,6,7,8-tetrahydro[1,2,4]triazolo[4,3-a]pyrazin-7-yl]-2-(2,4,5-trifluorophenyl)cyclohexyl]carbamate
-
486460-23-5
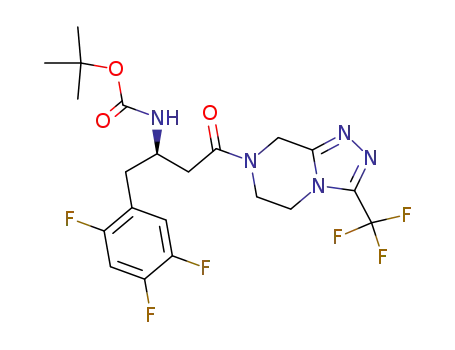
(R)-tert-butyl 4-oxo-4-(3-(trifluoromethyl)-5,6-dihydro-[1,2,4]triazolo[4,3-a]pyrazin-7(8H)-yl)-1-(2,4,5-trifluorophenyl)butan-2-ylcarbamate
-
959839-54-4
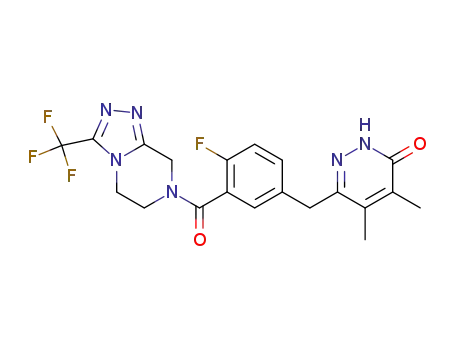
6-(4-Fluoro-3-{[3-(trifluoromethyl)-5,6-dihydro[1,2,4]triazolo[4,3-a]pyrazin-7(8H)-yl]carbonyl}benzyl)-4,5-dimethylpyridazin-3(2H)-one
Relevant Products
-
Ciprofloxacin Hydrochloride
CAS:86393-32-0
-
Anthraquinone
CAS:84-65-1
-
2,3,4,5,6-Bromopentafluorobenzene
CAS:344-04-7