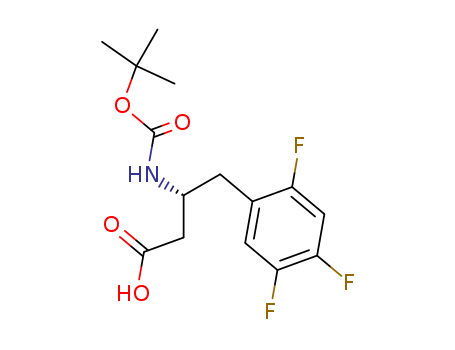
486460-00-8
- Product Name:(R)-3-(tert-butoxycarbonyl)-4-(2,4,5-trifluorophenyl)butanoic acid
- Molecular Formula:C15H18F3NO4
- Purity:99%
- Molecular Weight:333.307
Product Details;
CasNo: 486460-00-8
Molecular Formula: C15H18F3NO4
Top Purity 99% Chinese Manufacturer Supply (R)-3-(tert-butoxycarbonyl)-4-(2,4,5-trifluorophenyl)butanoic acid 486460-00-8 Competitive Price
- Molecular Formula:C15H18F3NO4
- Molecular Weight:333.307
- Vapor Pressure:1.25E-08mmHg at 25°C
- Melting Point:136-138℃
- Refractive Index:1.494
- Boiling Point:443.1 °C at 760 mmHg
- PKA:4.30±0.10(Predicted)
- Flash Point:221.8 °C
- PSA:75.63000
- Density:1.292 g/cm3
- LogP:3.40530
(R)-3-(tert-butoxycarbonyl)-4-(2,4,5-trifluorophenyl)butanoic acid (Cas 486460-00-8) Usage
|
Physical Form |
White to Yellow Solid |
|
Description |
(R)-3-(tert-butoxycarbonyl)-4-(2,4,5-trifluorophenyl)butanoic acid is an important intermediate for the preparation of sitagliptin. Sitagliptinphosphate is the first dipeptidase -IV(dpp-4) inhibitor approved by FDA in 2006. It is used for the treatment of type II diabetes mellitus. It has obvious hypoglycemic effect when used alone or in combination with metformin and pioglitazone, and it is safe to take, well tolerated, with few adverse reactions. |
|
Uses |
(R)-3-(tert-butoxycarbonyl)-4-(2,4,5-trifluorophenyl)butanoic acid has been used as a reactant for the preparation of dipeptidyl peptidase-4 (DPP4) inhibitors for the treatment of type 2 diabetes, such as sitagliptin. |
InChI:InChI=1/C15H18F3NO4/c1-15(2,3)23-14(22)19-9(6-13(20)21)4-8-5-11(17)12(18)7-10(8)16/h5,7,9H,4,6H2,1-3H3,(H,19,22)(H,20,21)/t9-/m1/s1
486460-00-8 Relevant articles
Application of the asymmetric hydrogenation of enamines to the preparation of a beta-amino acid pharmacophore
Kubryk, Michele,Hansen, Karl B.
, p. 205 - 209 (2006)
(3R)-3-[N-(tert-Butoxycarbonyl)amino]-4-...
Nickel-Catalyzed Asymmetric Hydrogenation for the Synthesis of a Key Intermediate of Sitagliptin
Sudhakaran, Shana,Shinde, Prasad G.,Aratikatla, Eswar K.,Kaulage, Sandeep H.,Rana, Priksha,Parit, Ratan S.,Kavale, Dattatry S.,Senthilkumar, Beeran,Punji, Benudhar
supporting information, (2021/12/09)
Nickel-catalyzed enantioselective hydrog...
Synthesis of (?)-(R)-Sitagliptin by RhI-Catalyzed Asymmetric Hydroamination
Berthold, Dino,Breit, Bernhard
, p. 6247 - 6249 (2021/09/25)
We report of a concise synthesis of (R)-...
Preparation method of 1-morpholinyl-4-(2,4,5-trifluorophenyl)butane-1,3-dione
-
Paragraph 0087-0088; 0091, (2020/05/02)
The invention discloses a preparation me...
486460-00-8 Process route
-
- 936630-57-8
(R)-3-amino-4-phenyl(2,4,5-trifluorophenyl)butanoic acid

-
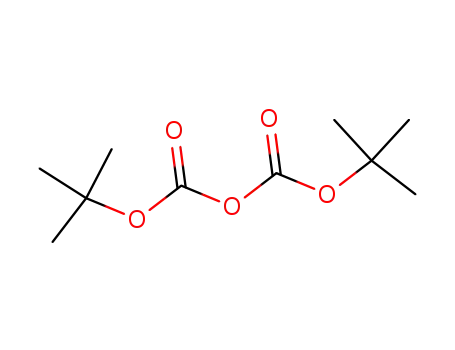
- 24424-99-5
di-tert-butyl dicarbonate

-
![(3R)-3-[(1,1-dimethylethoxycarbonyl)amino]-4-(2,4,5-trifluorophenyl)butanoic acid](/upload/2023/8/25176691-4e76-4bf3-9e1f-72e3e7888e7b.png)
- 486460-00-8,922178-94-7
(3R)-3-[(1,1-dimethylethoxycarbonyl)amino]-4-(2,4,5-trifluorophenyl)butanoic acid
| Conditions | Yield |
|---|---|
|
With triethylamine; In 1,4-dioxane; water; at 20 ℃;
|
91% |
|
(R)-3-amino-4-phenyl(2,4,5-trifluorophenyl)butanoic acid; With lithium hydroxide; In 1,4-dioxane; water; at 0 - 30 ℃;
di-tert-butyl dicarbonate; In 1,4-dioxane; water; at 25 - 30 ℃;
|
91% |
|
With sodium hydrogencarbonate; In tetrahydrofuran; water; at 20 ℃; for 24h;
|
91% |
|
With triethylamine; In dichloromethane; at 20 ℃; for 3h;
|
85% |
|
With triethylamine; In dichloromethane; at 20 ℃; for 10h;
|
81.7% |
|
With triethylamine; In dichloromethane; at 20 ℃; for 10h;
|
81.7% |
|
With triethylamine; In dichloromethane; at 20 ℃; for 10h;
|
81.7% |
|
In tetrahydrofuran;
|
60.6% |
|
With lithium hydroxide; In tetrahydrofuran; water; at 20 ℃; for 12h;
|
14 g |
|
With sodium hydrogencarbonate; In methanol; at 0 - 20 ℃; for 6h;
|
|
|
With sodium carbonate; In tetrahydrofuran; water; at 25 - 30 ℃;
|
30 g |
|
In water; at 20 ℃;
|
88.8 g |
|
With sodium hydroxide; In water; at 25 - 30 ℃;
|
|
|
With sodium hydroxide; In water; at 25 - 30 ℃;
|
-
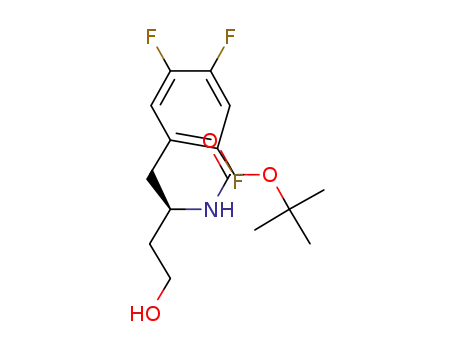
- 1048703-14-5
(R)-tert-butyl (4-hydroxy-1-(2,4,5-trifluorophenyl)butan-2-yl)carbamate

-
![(3R)-3-[(1,1-dimethylethoxycarbonyl)amino]-4-(2,4,5-trifluorophenyl)butanoic acid](/upload/2023/8/25176691-4e76-4bf3-9e1f-72e3e7888e7b.png)
- 486460-00-8,922178-94-7
(3R)-3-[(1,1-dimethylethoxycarbonyl)amino]-4-(2,4,5-trifluorophenyl)butanoic acid
| Conditions | Yield |
|---|---|
|
With sodium hypochlorite; sodium chlorite; disodium hydrogenphosphate; sodium dihydrogenphosphate; 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; In water; acetonitrile; at 38 ℃; for 0.333333h; Solvent;
|
98% |
|
With sodium hypochlorite; 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; sodium hydrogencarbonate; sodium bromide; In dichloromethane; at 0 ℃; for 2h;
|
90% |
|
(R)-tert-butyl (4-hydroxy-1-(2,4,5-trifluorophenyl)butan-2-yl)carbamate; With sodium hypochlorite; 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; sodium hydrogencarbonate; sodium bromide; In dichloromethane; at 0 ℃; for 2h;
With hydrogenchloride; In dichloromethane; water; pH=2 - 3;
|
90% |
|
With sodium hypochlorite; 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical;
|
90% |
486460-00-8 Upstream products
-
486460-25-7
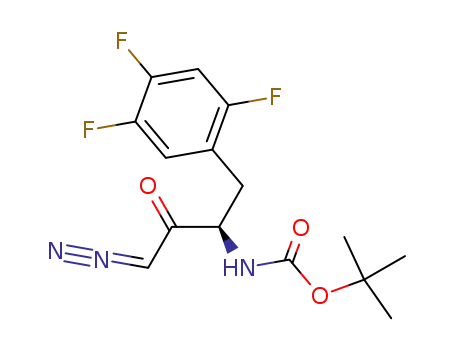
[3-diazo-2-oxo-1-(2,4,5-trifluoro-benzyl)-propyl]-carbamic acid tert-butyl ester
-
936630-57-8

(R)-3-amino-4-phenyl(2,4,5-trifluorophenyl)butanoic acid
-
24424-99-5

di-tert-butyl dicarbonate
-
881995-73-9
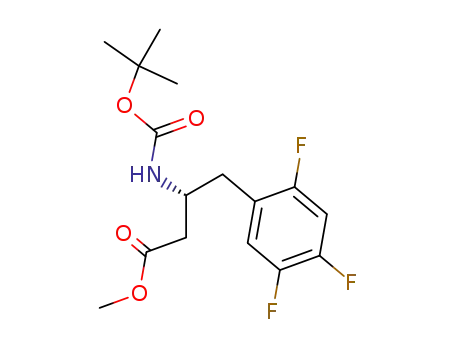
3-(R)-tert-butoxycarbonylamino-4-(2,4,5-trifluorophenyl)butyric acid methyl ester
486460-00-8 Downstream products
-
486460-23-5
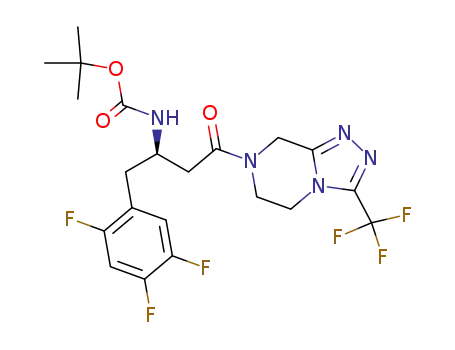
(R)-tert-butyl 4-oxo-4-(3-(trifluoromethyl)-5,6-dihydro-[1,2,4]triazolo[4,3-a]pyrazin-7(8H)-yl)-1-(2,4,5-trifluorophenyl)butan-2-ylcarbamate
-
1025933-74-7
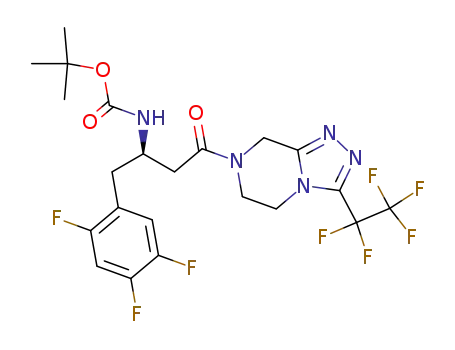
[3-oxo-3-(3-pentafluoroethyl-5,6-dihydro-8H-[1,2,4]triazolo[4,3-a]pyrazin-7-yl)-1-(2,4,5-trifluoro-benzyl)-propyl]-carbamic acid tert-butyl ester
-
1025950-29-1
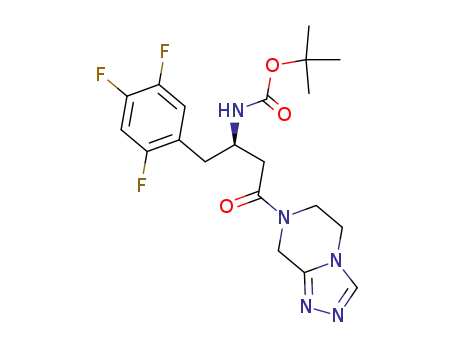
[3-(5,6-dihydro-8H-[1,2,4]triazolo[4,3-a]pyrazin-7-yl)-3-oxo-1-(2,4,5-trifluoro-benzyl)-propyl]-carbamic acid tert-butyl ester
-
892113-81-4
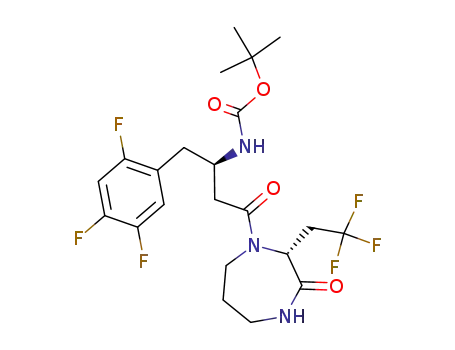
(3R)-4-[(3R)-3-(tert-butyloxycarbonylamino)-4-(2,4,5-trifluorophenyl) butanoyl]-3-(2,2,2-trifluoroethyl)-1,4-diazepin-2-one
Relevant Products
-
1,2,3-Trifluoro-4-nitrobenzene
CAS:771-69-7
-
Perindopril Arginine
CAS:612548-45-5
-
1,5-Dihydroxyanthraquinone
CAS:117-12-4