117-12-4
- Product Name:1,5-Dihydroxyanthraquinone
- Molecular Formula:C14H8O4
- Purity:99%
- Molecular Weight:240.215
Product Details;
CasNo: 117-12-4
Molecular Formula: C14H8O4
Quality Manufacturer Supply High Purity 1,5-Dihydroxyanthraquinone 117-12-4 Efficient Shipping
- Molecular Formula:C14H8O4
- Molecular Weight:240.215
- Vapor Pressure:8.21E-09mmHg at 25°C
- Melting Point:279 °C (dec.)(lit.)
- Refractive Index:1.732
- Boiling Point:452.7 °C at 760 mmHg
- PKA:6.42±0.20(Predicted)
- Flash Point:241.7 °C
- PSA:74.60000
- Density:1.54 g/cm3
- LogP:1.87320
1,5-Dihydroxyanthraquinone(Cas 117-12-4) Usage
|
Chemical Properties |
Yellow to Green Solid |
|
Uses |
1,5-Dihydroxyanthraquinone is an anthraquinone derivative with suppressive effects against nitric oxide (NO) synthase. Potential antimalarial agent. Used in studies as a potential competitive non-peptidic inhibitor of HIV-1 protei nase. |
|
Definition |
ChEBI: A dihydroxyanthraquinone that is anthracene-9,10-dione substituted by hydroxy groups at positions 1 and 5. |
|
Purification Methods |
Purify anthrarufin by column chromatography on silica gel with CHCl3/Et2O as eluent, followed by recrystallisation from acetone. Alternatively recrystallise it from glacial acetic acid [Flom & Barbara J Phys Chem 89 4489 1985]. [Beilstein 7 III 2359, 8 IV 3268.] |
InChI:InChI=1/C14H8O4/c15-9-5-1-3-7-11(9)14(18)8-4-2-6-10(16)12(8)13(7)17/h1-6,15-16H
117-12-4 Relevant articles
Efficient reductive Claisen rearrangement of prop-2’-enyloxyanthraquinones and 2’-chloroprop-2’-enyloxyanthraquinones with iron powder in ionic liquids
Nadali, Samaneh,Khoshroo, Ali,Aghapour, Ghasem
, p. 883 - 895 (2018)
A rapid and selective iron-mediated redu...
Surface-enhanced Raman scattering of 1,5-dihydroxyanthraquinone in silver sol
S.Edwin Jayaraj, V. Ramakrishnan
, Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy Volume 51, Issue 6, June 1995, Pages 979-983
Surface-enhanced Raman scattering studies on 1,5-dihydroxyanthraquinone (1,5-DHAQ) were undertaken to elucidate the molecular orientation on the silver particles and the enhancement mechanism. The 1,5-DHAQ molecule is adsorbed through its coordinating sites (CO groups) and it is found to have a stand-on configuration.
Highly selective three-step synthesis of rhein in chloroaluminate molten salt: preclusion of the Hayashi rearrangement
Gonnot, Vanessa,Antheaume, Cyril,Nicolas, Marc,Mioskowski, Charles,Baati, Rachid
experimental part, p. 6205 - 6210 (2010/03/24)
An expeditious, three-step synthesis of ...
Pathway of anthracene modification under simulated solar radiation
Mallakin, Ali,George Dixon,Greenberg, Bruce M.
, p. 1435 - 1441 (2007/10/03)
Exposure of polycyclic aromatic hydrocar...
117-12-4 Process route
-

- 87965-33-1
1,5-bis-diacetoxyboranyloxy-anthraquinone

-
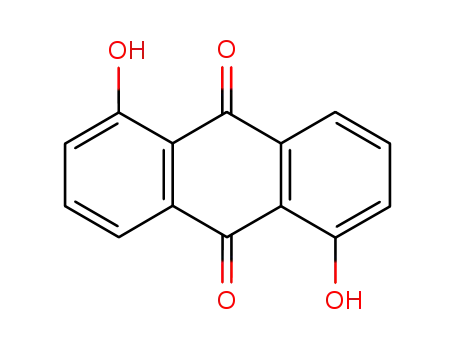
- 117-12-4
1,5-dihydroxyanthraquinone

-
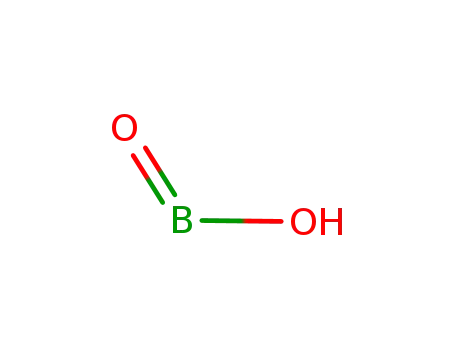
- 13460-50-9
metaboric acid

-
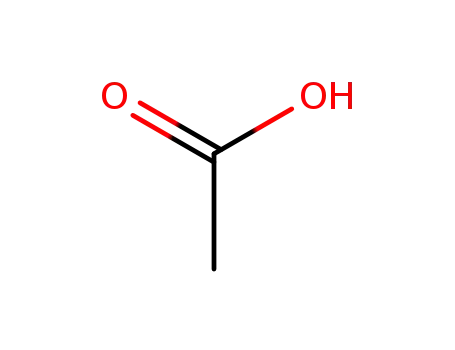
- 64-19-7,77671-22-8
acetic acid
| Conditions | Yield |
|---|---|
|
|
-
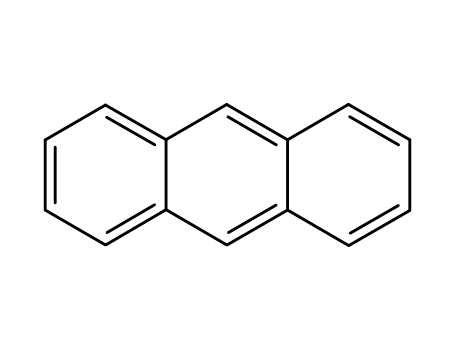
- 120-12-7
anthracene

-

- 117-12-4
1,5-dihydroxyanthraquinone

-
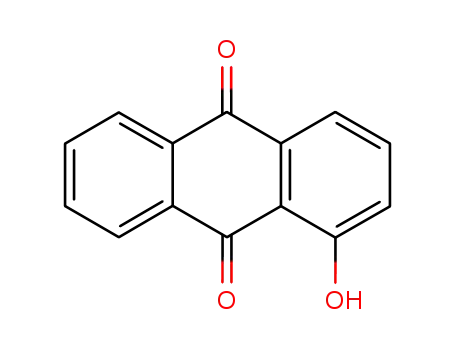
- 129-43-1
1-hydroxyanthraquinone

-

- 81-64-1,103220-12-8
1,4-dihydroxy-9,10-anthracenedione

-
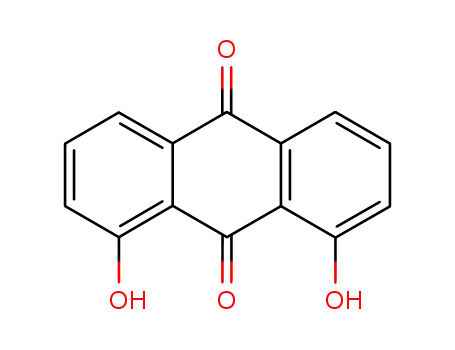
- 117-10-2
1,8-dihydroxy-9,10-anthracenedione
| Conditions | Yield |
|---|---|
|
In water; Further byproducts given; Formation of xenobiotics; simulated solar irradiation;
|
117-12-4 Upstream products
-
110-86-1
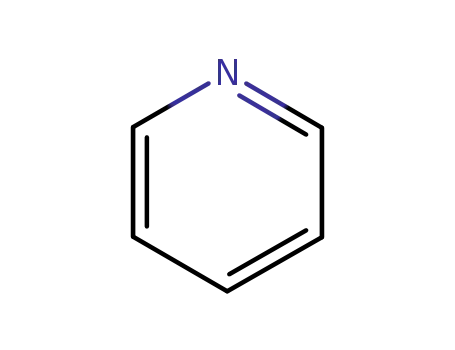
pyridine
-
82-35-9
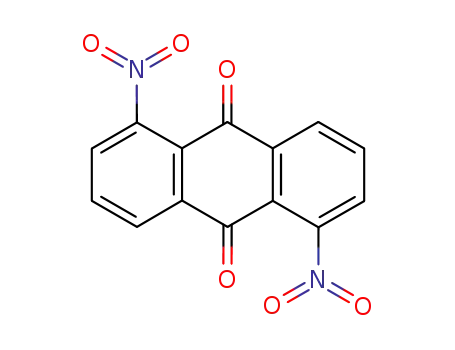
1.5-dinitroanthraquinone
-
1758-68-5
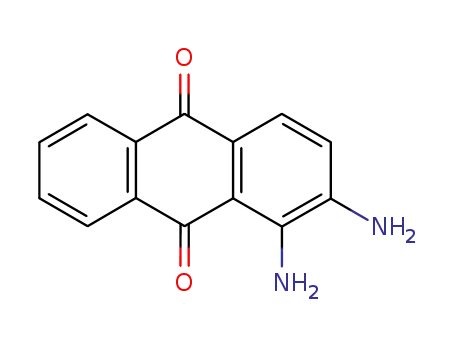
1,2-diamino-9,10-anthraquinone
-
117-14-6
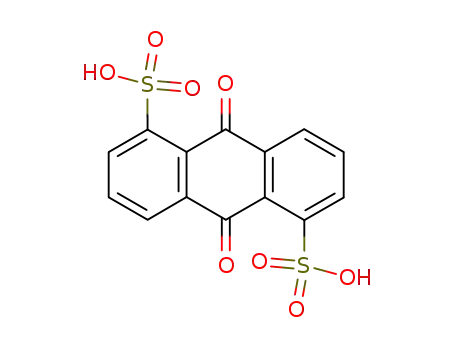
1,5-anthraquinone disulfonate
117-12-4 Downstream products
-
6448-90-4
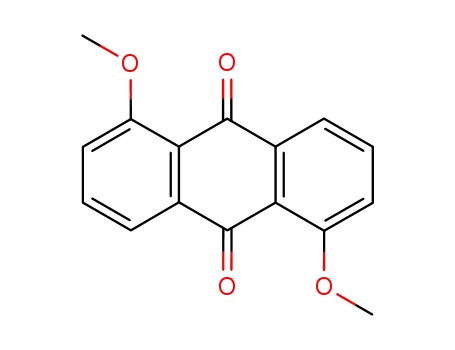
1,5-dimethoxy-anthraquinone
-
128-86-9

Alizarin Saphirol B
-
6492-85-9
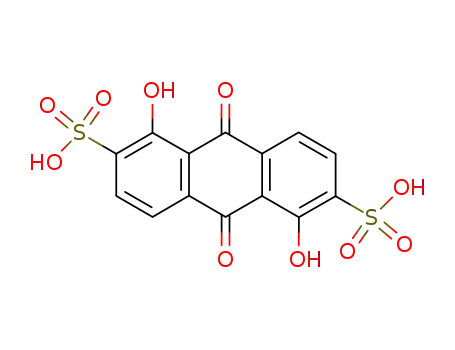
1,5-dihydroxyanthraquinone-2,6-disulfonic acid
-
128-91-6
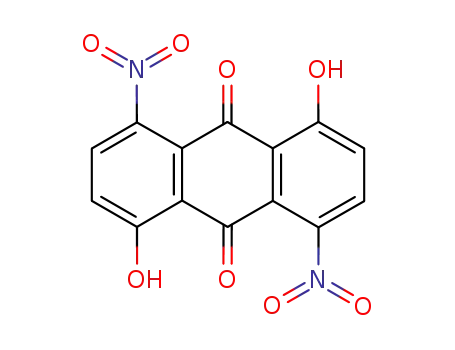
1,5-dihydroxy-4,8-dinitro-anthraquinone
Relevant Products
-
1,2,3-Trifluoro-4-nitrobenzene
CAS:771-69-7
-
Doxazosin
CAS:74191-85-8