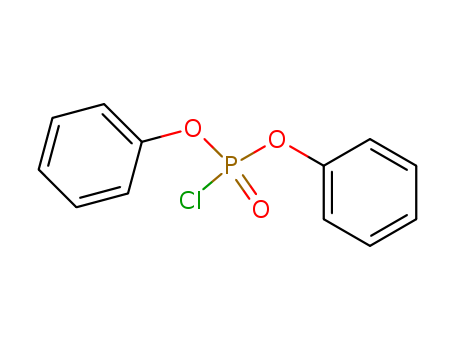
2524-64-3
- Product Name:Diphenyl chlorophosphate
- Molecular Formula:C12H10ClO3P
- Purity:99%
- Molecular Weight:268.636
Product Details;
CasNo: 2524-64-3
Molecular Formula: C12H10ClO3P
Appearance: clear colourless to light yellow liquid
99% Pure Factory Supply Diphenyl chlorophosphate 2524-64-3 In Stock
- Molecular Formula:C12H10ClO3P
- Molecular Weight:268.636
- Appearance/Colour:clear colourless to light yellow liquid
- Vapor Pressure:10 mm Hg ( 190 °C)
- Melting Point:314-316oC
- Refractive Index:n20/D 1.55(lit.)
- Boiling Point:363.5 °C at 760 mmHg
- Flash Point:256.2 °C
- PSA:45.34000
- Density:1.337 g/cm3
- LogP:4.49130
Diphenyl chlorophosphate(Cas 2524-64-3) Usage
|
Description |
Diphenyl chlorophosphate is a viscous colorless liquid. It can be prepared in situ by the reaction between diphenyl hydrogen phosphonate and carbon tetrachloride. It is used to prepare aromatic polyesters by polycondensation reaction in pyridine and polyesteramide. Diphenyl chlorophosphate is used as a phosphonating agent, such as to prepare phenylphosphonic acid. |
|
Uses |
Diphenyl chlorophosphate can be used to synthesize methadone, an opioid used for opioid maintenance therapy and for pain relief. It is used to control seedling grasses in turf. It undergoes anhydrous condensation with ethyl-4-bromo-butyrate and the hydrolysis product in alkaline medium of this condensation reaction is used in the quantitative determination of 3-cyano-3,3-diphenylpropionic acid. |
|
Reference |
F. Higashi, A. Hoshio, J. Kiyoshige, Preparation of aromatic polyesters by the direct polycondensation reaction with diphenyl chlorophosphate in pyridine, Polymer Chemistry, 1983, vol. 21, pp. 3241-3247 F. Higahi, M. Ozawa, A. Mochizuki, Synthesis of soluble aromatic polyesteramides by stepwise copolycondensation of bisphenols and aromatic diamines with diphenyl chlorophosphate in pyridine, 1986, vol. 24, pp.637-643 H. R. Allcock, M. A. Hofmann, C. M. Ambler, R. V. Morford, Phenylphosphonic Acid Functionalized Poly[aryloxyphosphazenes], 2002, vol. 35, pp. 3484-3489 |
|
Preparation |
The preparation of Diphenyl chlorophosphate is as follows:With a thermometer,Reflux condenser and mechanical agitation120 g of dichloromethane was placed in a 500 ml four-neck reaction flask.7.3 g of N,N-dimethylformamide,Stirring temperature control below 10 °C,Put 28g of bis(trichloromethyl) carbonate,A solution of 50 g of diphenyl phosphate and 120 g of dichloromethane was added.After the addition, the reaction was carried out for 3 hours. After the end of the reaction, the temperature was controlled below 10 ° C, 20 g of water was slowly added, and the mixture was stirred and the organic phase was dried over anhydrous sodium sulfate. The desiccant was removed by filtration, and the filtrate was evaporated under normal pressure. The temperature was lowered to 0 ° C, and the insoluble matter was removed by filtration to obtain 48.9 g of diphenyl chlorophosphate. The purity of GC was 99.2%, and the yield was 90.3%. |
|
General Description |
A clear colorless to light yellow liquid with a pungent odor. Insoluble in water and denser than water. Hence sinks in water. Contact may severely irritate skin, eyes and mucous membranes. Flash point above 235°F. |
|
Air & Water Reactions |
Insoluble in water. |
|
Reactivity Profile |
Diphenyl chlorophosphate is incompatible with bases (including amines) strong oxidizing agents, and alcohols. May react vigorously or explosively if mixed with diisopropyl ether or other ethers in the presence of trace amounts of metal salts [J. Haz. Mat., 1981, 4, 291]. May form highly toxic and flammable phosphine gas in the presence of strong reducing agents such as hydrides. Partial oxidation by oxidizing agents may result in the release of toxic phosphorus oxides. |
|
Health Hazard |
TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution. |
|
Fire Hazard |
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated. |
|
Purification Methods |
Fractionally distil it in 30 351.5490. a good vacuum; better use a spinning band column. [Walsh J Am Chem Soc 81 3023 1959, IR: Bellamy & Beecher J Chem Soc 475 1952, Beilstein 6 IV 737.] |
InChI:InChI=1/C12H10ClO2P/c13-16(14-11-7-3-1-4-8-11)15-12-9-5-2-6-10-12/h1-10H
2524-64-3 Relevant articles
Synthesis of flame-retardant phosphaphenanthrene derivatives with high phosphorus contents
Zheng, Jinyun,Yu, Yujian,Zhang, Lulu,Zhen, Xiaomin,Zhao, Yufen
, p. 1688 - 1692 (2014)
Two novel types of phosphate derivatives...
Zinc-catalyzed transformation of diarylphosphoryl azides to diarylphosphate esters and amides
Ying, Jun,Gao, Qian,Wu, Xiao-Feng
supporting information, p. 1540 - 1543 (2020/04/15)
We have developed a facile and efficient...
Chemical synthesis method of diphenyl chlorophosphate
-
Paragraph 0019; 0020, (2019/10/01)
The invention relates to a synthesis met...
Preparation method of cresyl diphenyl phosphate
-
Paragraph 0037; 0049-0050; 0054-0055; 0059-0064, (2019/11/21)
The invention relates to a preparation m...
Exploration of chiral Lewis acid Mg2+ catalysts in the synthesis of aryl organophosphate triesters from phosphorus oxychloride through a three-step, two-pot substitution sequence
Granger, Emily,Solomianko, Katarzyna,Young, Cori,Erb, Jeremy
, p. 1404 - 1408 (2018/03/13)
A variety of nucleophilic and Lewis acid...
2524-64-3 Process route
-

- 850-30-6
N-methylpyridinium diphenyl phosphate

-
- 10448-49-4
tetraphenyl pyrophosphate

-
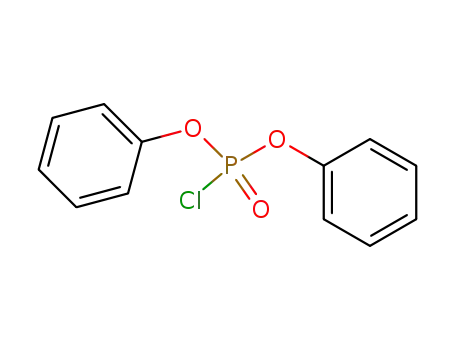
- 2524-64-3
chlorophosphoric acid diphenyl ester
| Conditions | Yield |
|---|---|
|
With phosgene; In dichloromethane; at 0 ℃; for 3h;
|
|
|
With phosgene; In dichloromethane; at -80 ℃; for 3h;
|
|
|
With phosgene; In dichloromethane; at -80 ℃; for 3h; Product distribution; var. addn. time; var. temp.;
|
-
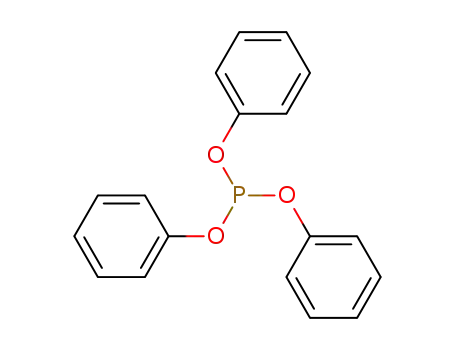
- 101-02-0
triphenyl phosphite

-
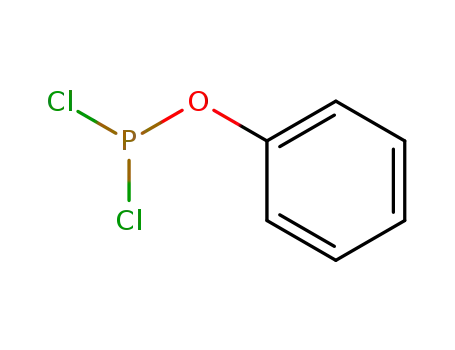
- 3426-89-9
phenyl phosphorodichloridite

-

- 2524-64-3
chlorophosphoric acid diphenyl ester
| Conditions | Yield |
|---|---|
|
With phosphorus trichloride; at 150 ℃; im Rohr;
|
2524-64-3 Upstream products
-
101-02-0

triphenyl phosphite
-
56-23-5
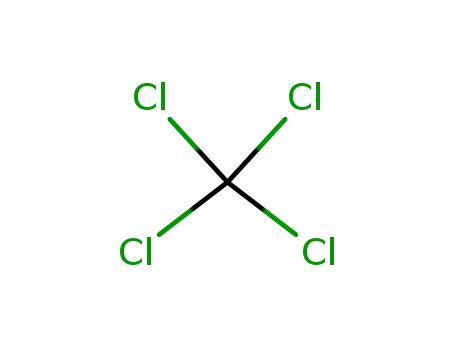
tetrachloromethane
-
3577-87-5
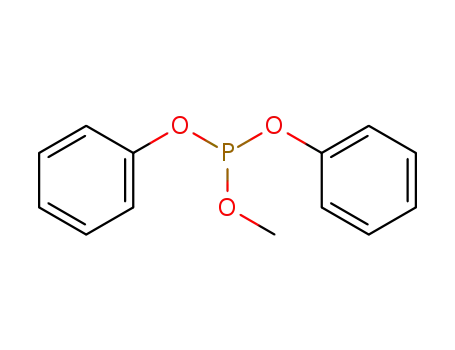
diphenyl methyl phosphite
-
5382-00-3
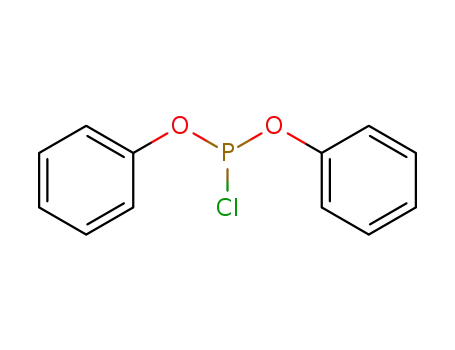
Diphenyl phosphorochloridite
2524-64-3 Downstream products
-
5314-06-7
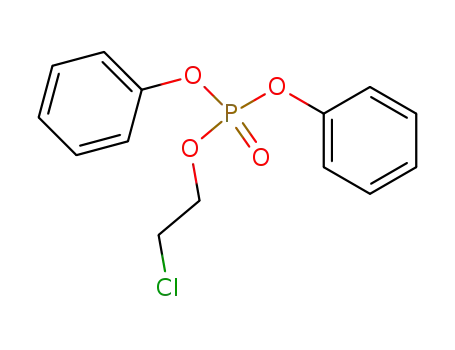
2-chloroethyl diphenyl phosphate
-
6214-09-1
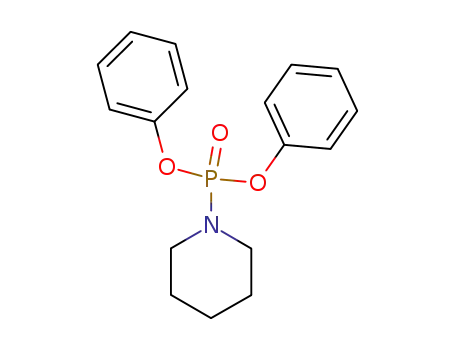
diphenyl piperidin-1-ylphosphonate
-
7412-25-1
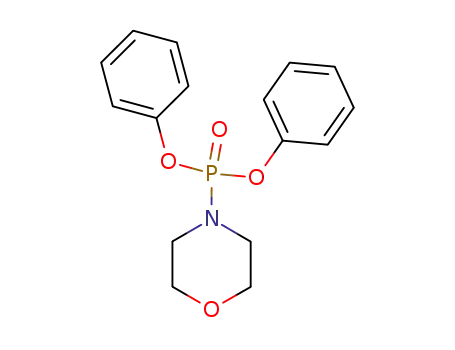
morpholinodiphenylphosphine oxide
-
109698-00-2
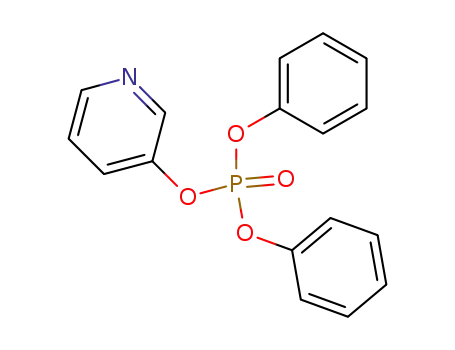
phosphoric acid diphenyl ester-[3]pyridyl ester
Relevant Products
-
phosphorylcholine
CAS:107-73-3
-
2,3-Difluoro-5-chloropyridin
CAS:89402-43-7
-
2-Fluorophenol
CAS:367-12-4