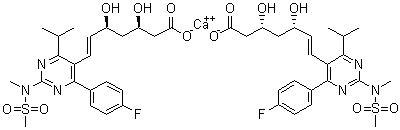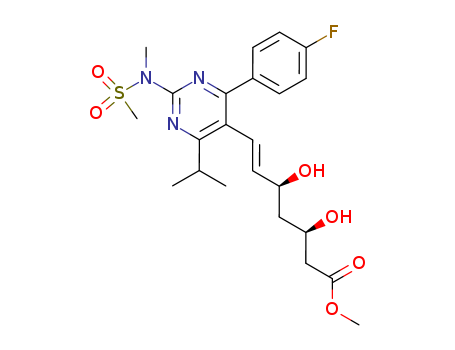
147118-40-9
- Product Name:Rosuvastatin methyl ester
- Molecular Formula:C23H30FN3O6S
- Purity:99%
- Molecular Weight:495.572
Product Details;
CasNo: 147118-40-9
Molecular Formula: C23H30FN3O6S
Best Quality Quality Factory Supply Rosuvastatin methyl ester 147118-40-9 Fast Shipping
- Molecular Formula:C23H30FN3O6S
- Molecular Weight:495.572
- Vapor Pressure:4E-20mmHg at 25°C
- Refractive Index:1.575
- Boiling Point:692.3 °C at 760 mmHg
- PKA:13.33±0.20(Predicted)
- Flash Point:372.5 °C
- PSA:138.30000
- Density:1.313 g/cm3
- LogP:3.57090
Rosuvastatin methyl ester(Cas 147118-40-9) Usage
|
Chemical Properties |
White Solid |
|
Uses |
Rosuvastatin Methyl Ester is an impurity of Rosuvastatin (R700500) in the pharmaceutical dosage forms under forced degradation conditions. |
|
Consumer Uses |
ECHA has no public registered data indicating whether or in which chemical products the substance might be used. ECHA has no public registered data on the routes by which this substance is most likely to be released to the environment. |
InChI:InChI=1/C23H30FN3O6S/c1-14(2)21-19(11-10-17(28)12-18(29)13-20(30)33-4)22(15-6-8-16(24)9-7-15)26-23(25-21)27(3)34(5,31)32/h6-11,14,17-18,28-29H,12-13H2,1-5H3/b11-10+/t17-,18-/m1/s1
147118-40-9 Relevant articles
Synthetic studies on statins. Part 3: A facile synthesis of rosuvastatin calcium through catalytic enantioselective allylation strategy
Chen, Xiaofei,Xiong, Fangjun,Zheng, Chen,Li, Jie,Chen, Fener
, p. 5794 - 5799 (2014)
A concise and stereocontrolled synthesis...
Statins intermediate and its derivatives
-
Paragraph 0022-0023, (2019/04/10)
The invention discloses a preparation me...
Preparation process of rosuvastatin calcium preparation
-
Paragraph 0034; 0042; 0046; 0050, (2018/10/11)
The invention provides a preparation pro...
Method for the preparation of high purity Rosuvastatin Calcium salt
-
Paragraph 0038; 0039, (2017/01/17)
The present invention relates to a metho...
3,5-dihydroxyhept-6-enoic acid derivative preparation method
-
Paragraph 0047 - 0050, (2017/04/19)
The invention relates to a preparation m...
147118-40-9 Process route
-
![2-[(4R,6S)-6-[(E)-2-[4-(4-fluorophenyl)-2-(N-methylmethanesulfonamido)-6-(isopropyl)pyrimidin-5-yl]vinyl]-2,2-dimethyl-1,3-dioxan-4-yl]acetic acid methyl ester](/upload/2023/8/8e7d26ff-6e0b-4a38-ad20-07f4be46f6fa.png)
- 851440-19-2
2-[(4R,6S)-6-[(E)-2-[4-(4-fluorophenyl)-2-(N-methylmethanesulfonamido)-6-(isopropyl)pyrimidin-5-yl]vinyl]-2,2-dimethyl-1,3-dioxan-4-yl]acetic acid methyl ester

-
(E)-7-[4-(4-fluorophenyl)-6-isopropyl-2-[methyl(methylsulfonyl)amino]pyrimidin-5-yl](3R,5S)-3,5-dihydroxyhept-6-enoic acid methyl ester
- 147118-40-9
(E)-7-[4-(4-fluorophenyl)-6-isopropyl-2-[methyl(methylsulfonyl)amino]pyrimidin-5-yl](3R,5S)-3,5-dihydroxyhept-6-enoic acid methyl ester
| Conditions | Yield |
|---|---|
|
With hydrogenchloride; In water; acetonitrile; at 40 ℃; for 8h;
|
83% |
|
With hydrogenchloride; In methanol; acetonitrile; at 20 - 25 ℃; for 8h;
|
79% |
|
With hydrogenchloride; In water; acetonitrile; at 20 - 25 ℃; for 8h;
|
62.7 g |
-
![methyl (3R,6E)-7-[4-(4-fluorophenyl)-2-(N-methylmethanesulfonamido)-6-(propan-2-yl)pyrimidin-5-yl]-3-hydroxy-5-oxohept-6-enoate](/upload/2023/8/25dff880-ad49-4c27-8b79-647c96bcd26b.png)
- 147118-39-6
methyl (3R,6E)-7-[4-(4-fluorophenyl)-2-(N-methylmethanesulfonamido)-6-(propan-2-yl)pyrimidin-5-yl]-3-hydroxy-5-oxohept-6-enoate

-
(E)-7-[4-(4-fluorophenyl)-6-isopropyl-2-[methyl(methylsulfonyl)amino]pyrimidin-5-yl](3R,5S)-3,5-dihydroxyhept-6-enoic acid methyl ester
- 147118-40-9
(E)-7-[4-(4-fluorophenyl)-6-isopropyl-2-[methyl(methylsulfonyl)amino]pyrimidin-5-yl](3R,5S)-3,5-dihydroxyhept-6-enoic acid methyl ester
| Conditions | Yield |
|---|---|
|
methyl (3R,6E)-7-[4-(4-fluorophenyl)-2-(N-methylmethanesulfonamido)-6-(propan-2-yl)pyrimidin-5-yl]-3-hydroxy-5-oxohept-6-enoate; With diethyl methoxy borane; In tetrahydrofuran; methanol; at -78 ℃; for 0.5h;
With sodium tetrahydroborate; In tetrahydrofuran; methanol; for 3h;
|
85.2% |
|
methyl (3R,6E)-7-[4-(4-fluorophenyl)-2-(N-methylmethanesulfonamido)-6-(propan-2-yl)pyrimidin-5-yl]-3-hydroxy-5-oxohept-6-enoate; With diethylmethoxyborane-THF; In tetrahydrofuran; methanol; at -78 ℃; for 0.5h;
With sodium tetrahydroborate; In tetrahydrofuran; methanol; for 3h;
With acetic acid; In tetrahydrofuran; methanol; pH=8;
|
85.2% |
|
With sodium tetrahydroborate; diethyl methoxy borane; Yield given. Multistep reaction; 1.) THF, MeOH, -78 deg C, 30 min, 2.) THF, MeOH, 3 h;
|
|
|
methyl (3R,6E)-7-[4-(4-fluorophenyl)-2-(N-methylmethanesulfonamido)-6-(propan-2-yl)pyrimidin-5-yl]-3-hydroxy-5-oxohept-6-enoate; With sodium tetrahydroborate; diethyl methoxy borane; In tetrahydrofuran; methanol; at -90 ℃;
With acetic acid; In tetrahydrofuran; methanol;
|
|
|
With methanol; sodium tetrahydroborate; diethyl methoxy borane; In tetrahydrofuran; at -90 - -80 ℃; Product distribution / selectivity; Inert atmosphere;
|
|
|
methyl (3R,6E)-7-[4-(4-fluorophenyl)-2-(N-methylmethanesulfonamido)-6-(propan-2-yl)pyrimidin-5-yl]-3-hydroxy-5-oxohept-6-enoate; With diethyl methoxy borane; In tetrahydrofuran; methanol; at -85 ℃; for 1.5h; Inert atmosphere;
With sodium tetrahydroborate; In tetrahydrofuran; methanol; at -85 - 30 ℃; for 12.5h;
With acetic acid; In tetrahydrofuran; methanol;
|
|
|
With methanol; sodium tetrahydroborate; diethyl methoxy borane; In tetrahydrofuran; at -90 - -80 ℃; for 6h; Inert atmosphere;
|
|
|
With sodium tetrahydroborate; diethyl methoxy borane; In tetrahydrofuran; methanol; at -105 - 95 ℃; Product distribution / selectivity;
|
|
|
With sodium tetrahydroborate; diethyl methoxy borane; In tetrahydrofuran; methanol; at -78 - -75 ℃; for 1.5h;
|
10.7 g |
|
methyl (3R,6E)-7-[4-(4-fluorophenyl)-2-(N-methylmethanesulfonamido)-6-(propan-2-yl)pyrimidin-5-yl]-3-hydroxy-5-oxohept-6-enoate; With diethyl methoxy borane; In tetrahydrofuran; methanol; at -85 - -80 ℃; Inert atmosphere;
With sodium tetrahydroborate; In tetrahydrofuran; methanol; at -85 - 30 ℃; Inert atmosphere;
|
6 g |
|
With methanol; sodium tetrahydroborate; diethyl methoxy borane; In tetrahydrofuran; at -80 - 25 ℃; for 8.5h; Inert atmosphere;
|
15.8 g |
|
methyl (3R,6E)-7-[4-(4-fluorophenyl)-2-(N-methylmethanesulfonamido)-6-(propan-2-yl)pyrimidin-5-yl]-3-hydroxy-5-oxohept-6-enoate; With diethyl methoxy borane; In tetrahydrofuran; methanol; at -78 ℃; for 3h; Inert atmosphere;
With methanol; sodium tetrahydroborate; In tetrahydrofuran; at -78 ℃; for 1h;
|
13 g |
147118-40-9 Upstream products
-
147118-39-6

methyl (3R,6E)-7-[4-(4-fluorophenyl)-2-(N-methylmethanesulfonamido)-6-(propan-2-yl)pyrimidin-5-yl]-3-hydroxy-5-oxohept-6-enoate
-
459-57-4
4-fluorobenzaldehyde
-
122930-45-4

(E)-2-<(4-fluorophenyl)methylene>-4-methyl-3-oxopentanoic acid ethyl ester
-
7152-15-0

ethyl 4-methyl-3-oxo-pentanoate
147118-40-9 Downstream products
-
851440-19-2

2-[(4R,6S)-6-[(E)-2-[4-(4-fluorophenyl)-2-(N-methylmethanesulfonamido)-6-(isopropyl)pyrimidin-5-yl]vinyl]-2,2-dimethyl-1,3-dioxan-4-yl]acetic acid methyl ester
-
287714-41-4

rosuvastatin
-
1353050-09-5
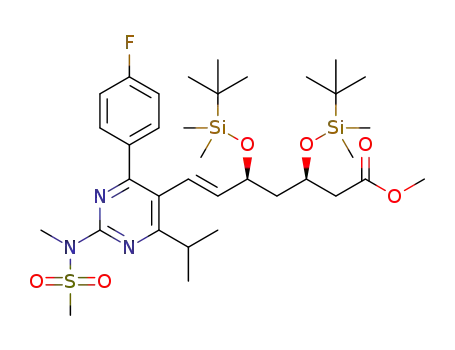
(3R,5S,E)-methyl 3,5-bis(tert-butyldimethylsilyloxy)-7-(4-(4-fluorophenyl)-6-isopropyl-2-(N-methylmethylsulfonamido)pyrimidin-5-yl)hept-6-enoate
-
1353050-10-8

(3R,5S,E)-3,5-bis(tert-butyldimethylsilyloxy)-7-(4-(4-fluorophenyl)-6-isopropyl-2-(N-methylmethylsulfonamido)pyrimidin-5-yl)hept-6-enoic acid
Relevant Products
-
Rosuvastatin calcium
CAS:147098-20-2
-
tert-Butyl rosuvastatin
CAS:355806-00-7
-
4-fluorobenzylamine
CAS:140-75-0