140-75-0
- Product Name:4-fluorobenzylamine
- Molecular Formula:C7H8FN
- Purity:99%
- Molecular Weight:125.146
Product Details;
CasNo: 140-75-0
Molecular Formula: C7H8FN
Appearance: Colorless to light yellow liquid
Best Quality Chinese Manufacturer Supply 4-fluorobenzylamine 140-75-0 Cheapest Price
- Molecular Formula:C7H8FN
- Molecular Weight:125.146
- Appearance/Colour:Colorless to light yellow liquid
- Vapor Pressure:0.134mmHg at 25°C
- Melting Point:183 °C
- Refractive Index:n20/D 1.512(lit.)
- Boiling Point:184.3 °C at 760 mmHg
- PKA:9.01±0.10(Predicted)
- Flash Point:73.3 °C
- PSA:26.02000
- Density:1.101 g/cm3
- LogP:1.98470
4-Fluorobenzylamine(Cas 140-75-0) Usage
|
Chemical Properties |
Colorless to light yellow liquid. |
|
Uses |
4-Fluorobenzylamine is used as a potassium channel blocking agent. It is used in the synthesis of new tris-iron(III) chelates of 3-hydroxy-4-pyridinone ligands. It reacts with the α- and γ-carboxyl groups of folic acid to yield 18F-labeled folate2. It is an important building block for the synthesis of 18F-labeled compounds. |
|
General Description |
[18F]4-fluorobenzylamine reacts with the α- and γ-carboxyl groups of folic acid to yield 18F-labeled folate. It is an important building block for the synthesis of 18F-labeled compounds. |
|
Synthesis |
In a 100ml reaction flask, add 25.5g (0.1mol) of N-(4-fluorobenzyl)phthalimide and 25ml of absolute ethanol, then add 6.3g (0.1mol) of 80% hydrazine hydrate solution, and heat Reflux 0.5g. Excessive hydrochloric acid was added to decompose the precipitated white precipitate, after cooling, an appropriate amount of water was added to stir, and suction filtered. The filtrate was neutralized with NaOH solution, extracted with ether, and the ether extract was dried, evaporated to remove ether, distilled under reduced pressure, and collected fractions at 44-46°C (67kPa) to obtain 7.1 g of 4-fluorobenzylamine, with a yield of 56.5%. |
InChI:InChI=1/C7H3F2NS/c8-5-1-2-7(10-4-11)6(9)3-5/h1-3H
140-75-0 Relevant articles
A mild and selective Cu(II) salts-catalyzed reduction of nitro, azo, azoxy, N-aryl hydroxylamine, nitroso, acid halide, ester, and azide compounds using hydrogen surrogacy of sodium borohydride
Kalola, Anirudhdha G.,Prasad, Pratibha,Mokariya, Jaydeep A.,Patel, Manish P.
supporting information, p. 3565 - 3589 (2021/10/12)
The first mild, in situ, single-pot, hig...
Synthesis method of p-fluorobenzylamine
-
Paragraph 0006; 0013; 0016-0018; 0021-0023; 0026-0027, (2021/06/13)
The invention discloses a synthesis meth...
Method for preparing primary amine by catalytically reducing nitrile compounds through nano-porous palladium catalyst
-
Paragraph 0089-0092, (2021/05/29)
The invention belongs to the technical f...
Zirconium-hydride-catalyzed site-selective hydroboration of amides for the synthesis of amines: Mechanism, scope, and application
Han, Bo,Jiao, Haijun,Wu, Lipeng,Zhang, Jiong
, p. 2059 - 2067 (2021/09/02)
Developing mild and efficient catalytic ...
140-75-0 Process route
-
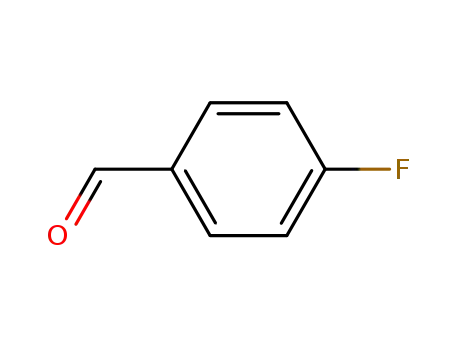
- 459-57-4
4-fluorobenzaldehyde

-
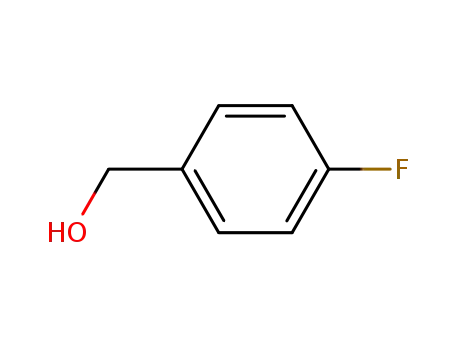
- 459-56-3
4-fluorobenzylic alcohol

-
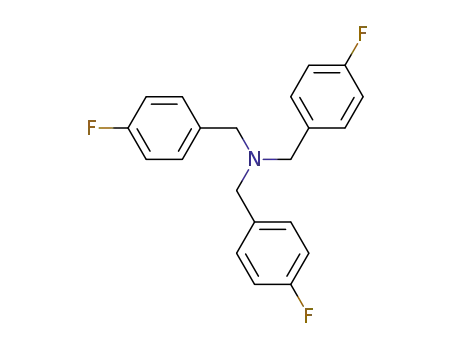
- 932724-63-5
tris(4-fluorobenzyl)amine

-
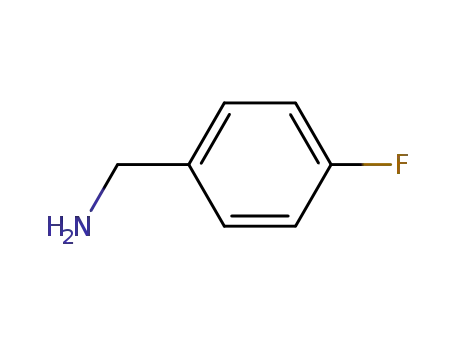
- 140-75-0
para-fluorobenzylamine

-
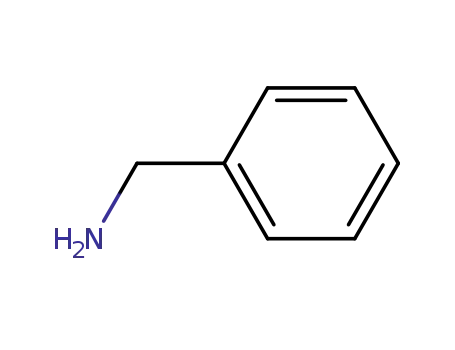
- 100-46-9
benzylamine

-
![1-(4-fluorophenyl)-N-[(4-fluorophenyl)methyl]methanamine](/upload/2023/8/314eb8e0-7463-4de6-92c2-de4e4dc6065d.png)
- 134227-41-1
1-(4-fluorophenyl)-N-[(4-fluorophenyl)methyl]methanamine
| Conditions | Yield |
|---|---|
|
With ammonia; hydrogen; Ni/C catalyst; In water; at 120 ℃; for 17.5h; under 28502.9 - 33753.4 Torr;
|
90.7% 0.4% 1.2% 1.8% 1.4% |
-
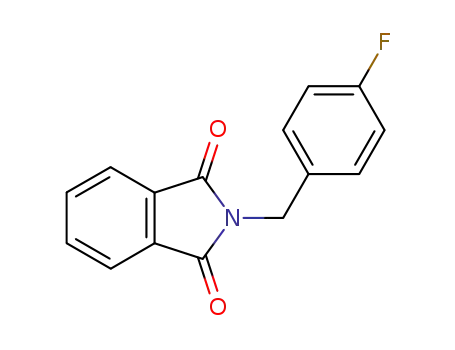
- 318-49-0
2-(4-fluorobenzyl)isoindoline-1,3-dione

-
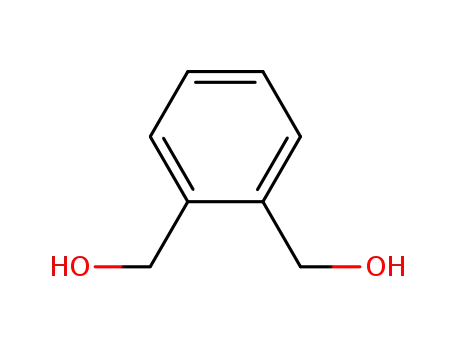
- 612-14-6
phthalyl alcohol

-

- 140-75-0
para-fluorobenzylamine
| Conditions | Yield |
|---|---|
|
With [Ru(PtBuNNHtBu)H(CO)Cl]; potassium tert-butylate; hydrogen; In tetrahydrofuran; at 110 ℃; for 24h; under 15001.5 Torr; Autoclave;
|
|
|
With C25H19BrMnN2O2P; potassium tert-butylate; hydrogen; In tetrahydrofuran; at 130 ℃; for 48h; under 22502.3 Torr; Inert atmosphere; Glovebox; Autoclave; Green chemistry;
|
84 %Spectr. 89 %Spectr. |
140-75-0 Upstream products
-
100-97-0
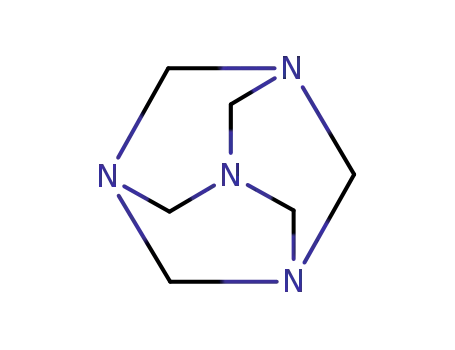
hexamethylenetetramine
-
352-11-4
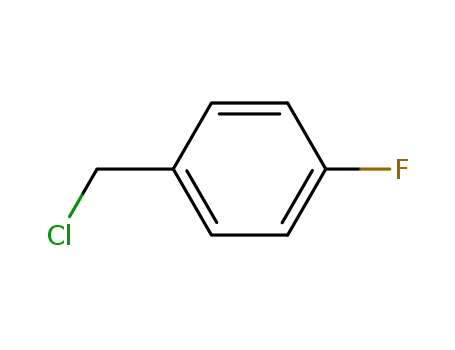
1-chloromethyl-4-fluorobenzene
-
1194-02-1
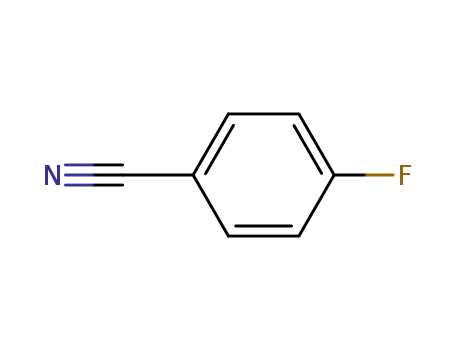
4-fluorobenzonitrile
-
318-49-0

2-(4-fluorobenzyl)isoindoline-1,3-dione
140-75-0 Downstream products
-
1692-02-0
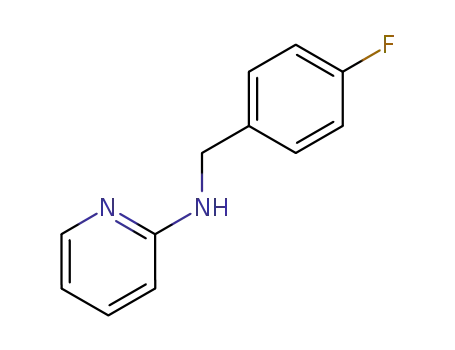
N-(4-fluorobenzyl)pyridine-2-amine
-
2714-80-9
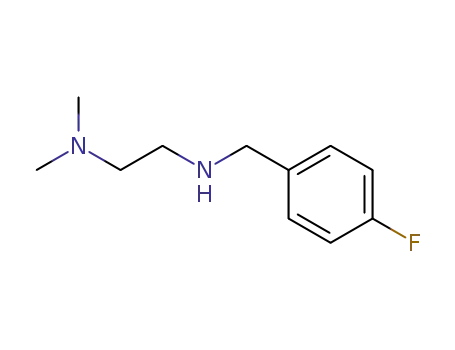
N-(4-fluorobenzyl)-N',N'-dimethylethylenediamine
-
97484-91-8
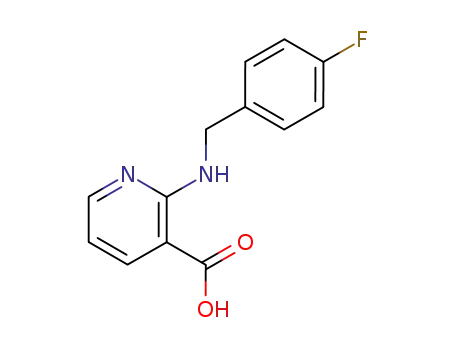
2-<(4-fluorophenyl)methylamino>-3-pyridinecarboxylic acid
-
73733-74-1
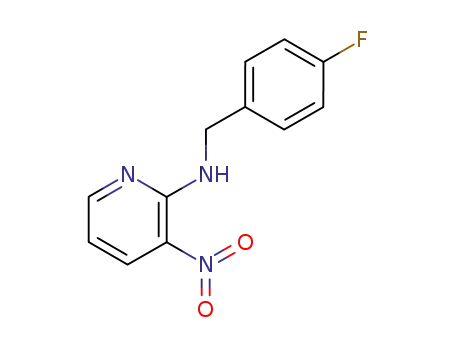
N-<(4-fluorophenyl)methyl>-3-nitro-2-pyridinamine
Relevant Products
-
1,2,3-Trifluoro-4-nitrobenzene
CAS:771-69-7
-
Rosuvastatin methyl ester
CAS:147118-40-9
-
Ofloxacin Q Acid
CAS:82419-35-0