5579-84-0
- Product Name:Betahistine dihydrochloride
- Molecular Formula:C8H12N2.2ClH
- Purity:99%
- Molecular Weight:209.119
Product Details;
CasNo: 5579-84-0
Molecular Formula: C8H12N2.2ClH
Appearance: white to light yellow crystalline powder
Chinese Factory Supply Betahistine dihydrochloride 5579-84-0, Buy with Low Price
- Molecular Formula:C8H12N2.2ClH
- Molecular Weight:209.119
- Appearance/Colour:white to light yellow crystalline powder
- Vapor Pressure:0.187mmHg at 25°C
- Melting Point:150-154 °C
- Boiling Point:210.9 °C at 760 mmHg
- Flash Point:96.7 °C
- PSA:24.92000
- Density:0.967 g/cm3
- LogP:2.83840
Betahistine dihydrochloride(Cas 5579-84-0) Usage
|
Description |
Betahistine dihydrochloride (BH.2HCl) is an orally active histamine analog which has been used to control vertigo, lack of hearing and tinnitus related to Ménière’s disease. The mechanism of BH.2HCl is to reduce the pressure of the membranous labyrinth that results in enhancement of the microvasculature circulation and improves the signs of Ménière’s disease. Peroral administration undergoes extensive first-pass metabolism and gastric irritation in patients with peptic ulcer. In treatment of vertigo, a uniform and constant supply of drug is required in order to maintain steady-state concentration of the drug in the body. Unfortunately, BH.2HCl possesses a short half-life of about 3–4 h and requires frequent administration of the drug,12–15 thereby making it an ideal candidate for controlled release preparations. |
|
Chemical Properties |
white to light yellow crystalline powder |
|
Uses |
Betahistine is belongs to a group of medications used to treat vertigo associated with Ménière's disease. Vertigo is a condition that causes sufferers to have a sensation of rotation or movement of themselves or their surroundings. Ménière's disease is a disorder of the inner ear that causes vertigo in addition to symptoms such as ringing in the ears, headache, and loss of hearing.Betahistine is used to reduce the number of episodes of vertigo associated with Ménière's disease. It is believed to work by decreasing the pressure in the ear. This pressure is believed to contribute to the sense of dizziness, nausea, and ringing in the ears and hearing loss that people with Ménière's disease experience. |
|
Brand name |
Serc (Unimed). |
|
General Description |
Pharmaceutical secondary standards for application in quality control provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards |
|
Clinical Use |
Treatment of vertigo, tinnitus and hearing loss associated with Ménière’s syndrome |
|
Side effects |
Stomach upset, nausea, and headache may occur. This medication may also rarely cause drowsiness. If any of these effects persist or worsen, tell your doctor or pharmacist promptly.Remember that your doctor has prescribed this medication because he or she has judged that the benefit to you is greater than the risk of side effects. Many people using this medication do not have serious side effects.A very serious allergic reaction to this drug is rare. However, seek immediate medical attention if you notice any symptoms of a serious allergic reaction, including:rashitching/swelling (especially of the face/tongue/throat)severe dizzinesstrouble breathingThis is not a complete list of possible side effects. If you notice other effects not listed above, contact your doctor or pharmacist. |
|
Drug interactions |
Potentially hazardous interactions with other drugs None known |
|
Metabolism |
Betahistine is excreted almost exclusively in the urine as 2-pyridylacetic acid within 24 hours of administration. No unchanged betahistine has been detected. |
InChI:InChI=1/C8H12N2.2ClH/c1-9-7-5-8-4-2-3-6-10-8;;/h2-4,6,9H,5,7H2,1H3;2*1H
5579-84-0 Relevant articles
Preparation method of orthographic optimizing betahistine hydrochloride
-
Paragraph 0009, (2018/07/30)
The invention discloses a preparation me...
Aza-Michael-type addition reaction catalysed by a supported ionic liquid phase incorporating an anionic heteropoly acid
Ghasemi, Mohammad Hadi,Kowsari, Elaheh,Shafiee, Abbas
supporting information, p. 1150 - 1153 (2016/03/09)
In this work, we have obtained substitut...
5579-84-0 Process route
-
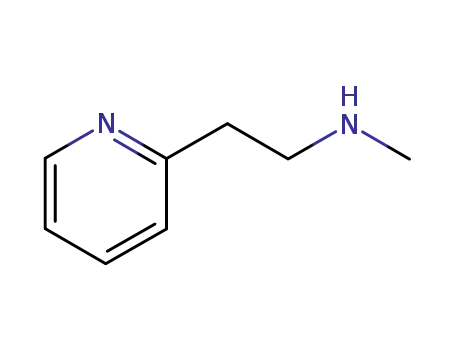
- 5638-76-6
betahistine

-
![[3H]-Betahistine dihydrochloride](/upload/2023/8/50176af3-4f70-4cb9-9792-c5c97f6cd5a6.png)
- 5579-84-0,15430-48-5
[3H]-Betahistine dihydrochloride
| Conditions | Yield |
|---|---|
|
With hydrogenchloride; In isopropyl alcohol; pH=2;
|
|
|
With hydrogenchloride; In water; isopropyl alcohol; at 0 - 10 ℃; pH=2;
|
-
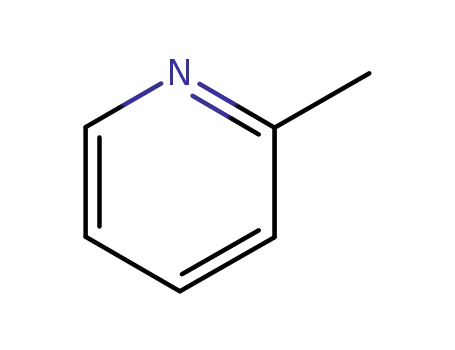
- 109-06-8
α-picoline

-
![[3H]-Betahistine dihydrochloride](/upload/2023/8/50176af3-4f70-4cb9-9792-c5c97f6cd5a6.png)
- 5579-84-0,15430-48-5
[3H]-Betahistine dihydrochloride
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 4 steps
1: 20 h / 125 °C / 3040.2 Torr / Inert atmosphere
2: sodium hydroxide / 2 h / 95 - 100 °C
3: hydrogenchloride / water / 95 - 105 °C
4: hydrogenchloride / water; isopropyl alcohol / 0 - 10 °C / pH 2
With hydrogenchloride; sodium hydroxide; In water; isopropyl alcohol;
|
5579-84-0 Upstream products
-
5638-76-6

betahistine
-
109-06-8

α-picoline
-
103-74-2
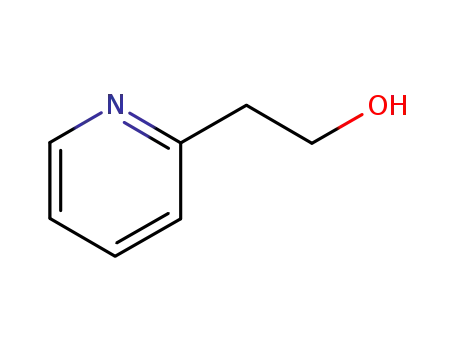
2-(2-Hydroxyethyl)pyridine
5579-84-0 Downstream products
-
1032445-64-9
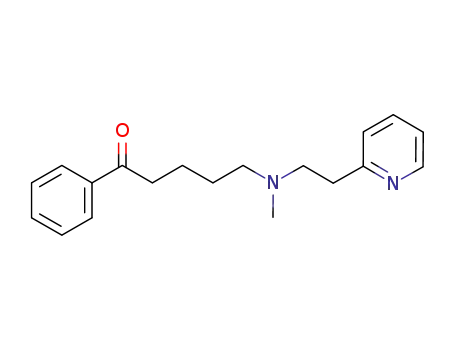
C19H24N2O
Relevant Products
-
Vonoprazan N -1
CAS:881677-11-8
-
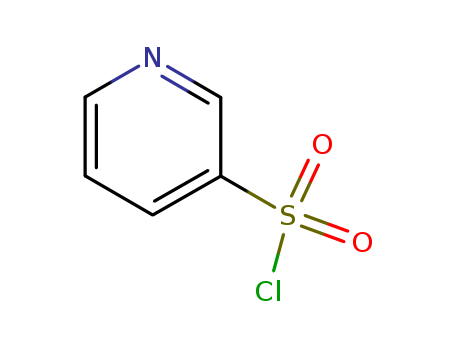
Pyridine-3-sulfonyl chloride
CAS:16133-25-8